Introduction
Atoms and molecules are the fundamental building blocks of matter. A clear understanding of atoms and molecules explains why substances combine in particular ways, why physical and chemical properties differ from one substance to another, and how new substances are formed in chemical reactions.
- Maharishi Kanad and Pakudha Katyayama in ancient India proposed that matter can be divided into smaller indivisible particles called Parmanu.
- Democritus and Leucippus in ancient Greece proposed a similar idea: matter is made of indivisible particles called atoms.
- These early ideas were philosophical and lacked experimental proof until modern chemistry developed in the 18th century.
- In the late 18th century, Antoine L. Lavoisier established quantitative methods in chemistry and laid foundations for modern chemical science by formulating laws about chemical combinations.
- Lavoisier and Joseph L. Proust performed careful experiments that led to two important laws: the Law of Conservation of Mass and the Law of Constant Proportions (also called the Law of Definite Proportions).
- These laws guided later work and helped John Dalton formulate his atomic theory that explained why these laws hold true for chemical reactions.
Try yourself:
Who proposed the idea that matter can be divided into smaller particles called Parmanu?
- A.Maharishi Kanad
- B.Democritus
- C.Antoine L. Lavoisier
- D.Joseph L. Proust
Laws of Chemical Combination
Two fundamental laws describe how substances combine in chemical reactions: the Law of Conservation of Mass and the Law of Constant Proportions. These laws provide the experimental basis for the atomic view of matter.
1. Law of Conservation of Mass
The Law of Conservation of Mass states that mass can neither be created nor destroyed in a chemical reaction. The total mass of reactants equals the total mass of products.
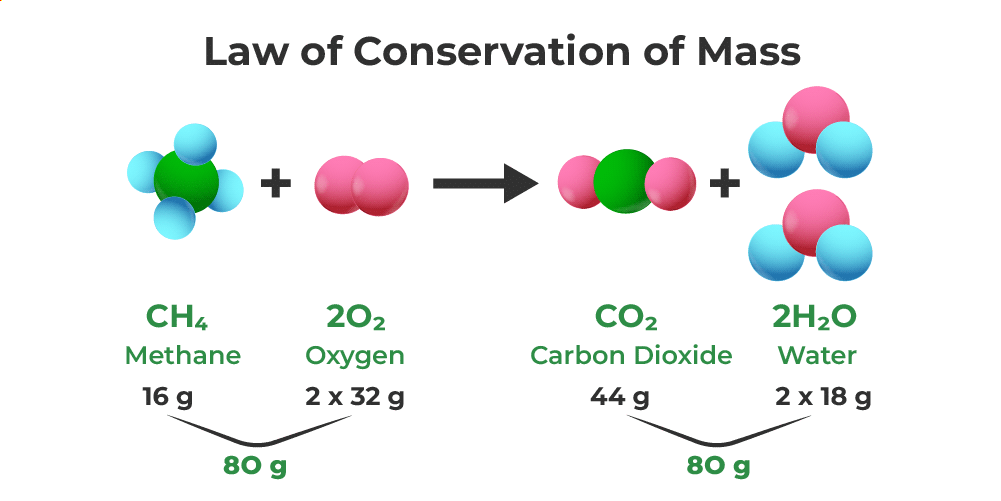 Example of Law of conservation of Mass
Example of Law of conservation of Mass
Careful experiments – for example, mixing chemical solutions in closed containers and measuring mass before and after reaction – show that the measured total mass remains unchanged, supporting this law.
Try yourself:
According to the Law of Conservation of Mass, what happens to the mass during a chemical reaction?
- A.The mass increases.
- B.The mass decreases.
- C.The mass remains constant.
- D.The mass is converted into energy.
2. Law of Constant Proportion
The Law of Constant Proportion (Law of Definite Proportions) states that a chemical compound always contains the same elements in the same fixed proportion by mass, irrespective of its source.
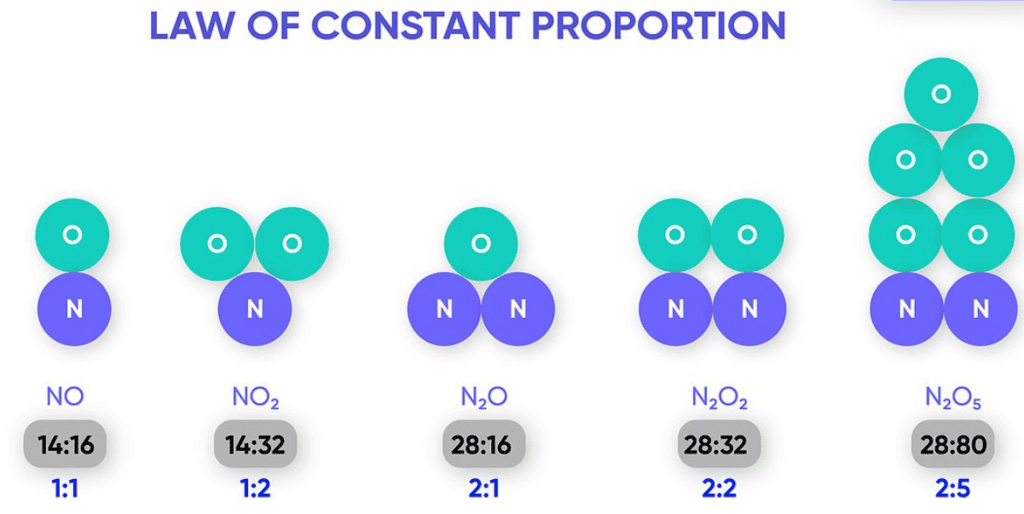
For example, pure water always contains hydrogen and oxygen in the mass ratio 1 : 8. This proportion is the same whether the water comes from a river, a well, or rain.
John Dalton’s Atomic Theory
To explain these laws, John Dalton proposed an atomic theory which gave an experimental and conceptual basis for atoms and compounds.
 John Dalton
John Dalton
Postulates of Dalton’s Atomic Theory
- All matter is made of extremely small particles called atoms.
- Atoms are indivisible by chemical means and remain unchanged in chemical reactions.
- Atoms of the same element are identical in mass and properties.
- Atoms of different elements have different masses and properties.
- Atoms combine in simple whole-number ratios to form compounds.
- The relative number and types of atoms in a given compound are constant (fixed composition).
Background on John Dalton
- John Dalton was born in 1766. His atomic hypothesis explained the Law of Conservation of Mass and the Law of Definite Proportions quantitatively and conceptually.
What is an Atom?
An atom is the smallest particle of an element that retains the chemical properties of that element and cannot be broken down by chemical means.
- Atoms are extremely small; a very large number of atoms are required to form visible matter.
- A layer only a few million atoms thick may be comparable in thickness to a sheet of paper.
Atomic Radius
The atomic radius is a measure of the size of an atom, typically expressed in nanometres (nm), where 1 nm = 10-9 m.
Try yourself:
Which statement best describes the Law of Constant Proportion?
- A.It states that in a chemical substance, elements are always present in definite proportions by volume.
- B.It states that in a chemical substance, elements are always present in definite proportions by mass.
- C.It states that in a chemical substance, elements are always present in indefinite proportions by mass.
- D.It states that in a chemical substance, elements can be present in any proportions by mass.
Modern Symbols of Elements
Element symbols evolved from early pictorial symbols to the simple one- or two-letter symbols used today. The International Union of Pure and Applied Chemistry (IUPAC) standardises these symbols.

- Historical background: John Dalton first used symbols to represent atoms. Later, Berzelius proposed using one or two letters derived from the element name to represent elements.
- Origin of element names: Some names come from places (e.g., copper from Cyprus) or from colours and other properties.
- Modern symbols: Most element symbols are derived from their English names; the first letter is capitalised and a second letter, if used, is lowercase.
- First letter + another letter: Examples include Chlorine: Cl, Zinc: Zn.
- Names from other languages: Some symbols come from Latin, Greek or other languages: Iron: Fe (ferrum), Sodium: Na (natrium), Potassium: K (kalium).

Symbols for Some Elements
Try yourself:
Which scientist pioneered the use of symbols for elements?
- A.Berzelius
- B.Dalton
- C.IUPAC
- D.None of the above
Atomic Mass
Atomic mass of an atom is the mass of that atom expressed relative to a standard. The standard used internationally is defined as 1/12 of the mass of a carbon-12 atom. The unit for atomic mass is the unified atomic mass unit (symbol u, also called amu).
- Atomic mass is the combined mass of protons, neutrons and electrons in an atom; in practice the electron mass is very small relative to protons and neutrons and often neglected in simple calculations.
- Atomic masses reported on the periodic table are average values that reflect the natural isotopic composition of the element.
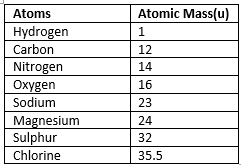 Atomic mass of some elements
Atomic mass of some elements
How Do Atoms Exist?
- Many atoms do not exist freely under normal conditions; they combine to form molecules or form ions that aggregate into ionic structures.
- Visible matter is composed of huge numbers of molecules or ionic units assembled together.
What is a Molecule?
A molecule is the smallest particle of an element or compound that can exist independently and retain the chemical properties of that substance.
Molecules of Elements
- Monoatomic molecules: Some elements exist as single atoms (monoatomic) in their natural gaseous state, e.g., Helium (He), Argon (Ar).
- Diatomic molecules: Several non-metals exist as molecules of two atoms, e.g., Hydrogen (H2), Oxygen (O2), Nitrogen (N2), Chlorine (Cl2).
- Polyatomic molecules: Some elements form molecules with more than two atoms, e.g., Phosphorus (P4), Sulphur (S8).
Try yourself:What is the atomic mass of an atom?
- A. The total mass of the neutrons and protons in an atom.
- B.The mass of a carbon-12 atom in its ground state.
- C.The average mass of a group of atoms.
- D.The mass of an atomic particle.
Atomicity
Atomicity is the number of atoms present in one molecule of an element.
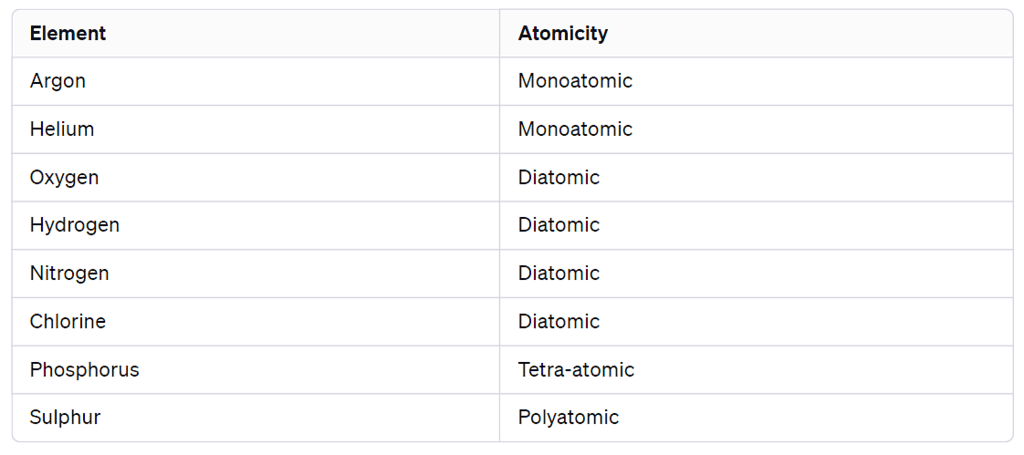 Atomicity of some non-metals
Atomicity of some non-metals
Molecules of Compounds
When atoms of different elements combine, they form molecules of compounds. These molecules have fixed compositions and properties different from their constituent elements.

What is an Ion?
- An ion is an atom or a group of atoms that carries an electric charge due to loss or gain of electrons.
- A positively charged ion is called a cation; a negatively charged ion is called an anion.
- Compounds formed from metals and non-metals often contain ions; such compounds are called ionic compounds.
- Polyatomic ions are groups of atoms bonded together that carry a net charge, for example, NO3–, SO42-, OH–.
Writing Chemical Formulae
- The chemical formula of a compound shows which elements are present and the number of atoms of each element in the smallest unit of that compound.
- To write formulae, you must know element symbols and the valencies (combining capacities) or ionic charges of the atoms/ions involved.
- Valency indicates how many electrons an atom gains, loses or shares when it forms a compound.
- Think of valency as the number of bonds an atom typically forms: it is the atom’s “combining power”.

Rules for Formula Writing
- The total positive charge and total negative charge in a neutral compound must balance.
- When writing formulae for compounds of a metal and a non-metal, write the metal first and the non-metal second (e.g., CaO, NaCl).
- Use the simplest whole-number ratio of atoms or ions that balances charges.
- When polyatomic ions are present in more than one number, enclose the polyatomic ion in brackets and write the number outside the brackets, e.g., Mg(OH)2.
- Practice with examples to become familiar with common valencies and formulas.
Try yourself:What is the atomicity of a molecule?
- A.The number of atoms in a molecule
- B.The number of ions in a molecule
- C.The number of elements in a molecule
- D.The number of protons in a molecule
| Also read: Short and Long Answer Questions: Atoms and Molecules |
Formulae of Simple Compounds
Binary compounds (formed by two elements) can be written by criss-crossing valencies or balancing charges of ions.
Example:
- Carbon tetrachloride, CCl4: Carbon (valency 4) combines with chlorine (valency 1) to give the formula CCl4.

- Magnesium chloride, MgCl2: Magnesium (valency 2) combines with chlorine (valency 1) to give MgCl2.

Molecular Mass
The molecular mass (relative molecular mass) of a molecule is the sum of the atomic masses of all atoms present in the molecule. It is expressed in atomic mass units (u).
Example 1:
(a) Calculate the relative molecular mass of water (H2O).
(b) Calculate the molecular mass of HNO3.
Solution:
(a)
Atomic mass of hydrogen = 1 u.
Atomic mass of oxygen = 16 u.
The molecular mass of H2O = 2 × (atomic mass of H) + 1 × (atomic mass of O).
The molecular mass of H2O = 2 × 1 + 16 = 18 u.
(b)
Atomic mass of hydrogen = 1 u.
Atomic mass of nitrogen = 14 u.
Atomic mass of oxygen = 16 u.
The molecular mass of HNO3 = 1 × (atomic mass of H) + 1 × (atomic mass of N) + 3 × (atomic mass of O).
The molecular mass of HNO3 = 1 + 14 + 3 × 16 = 63 u.
Try yourself:What is the chemical formula for magnesium chloride?
- A.MgC2
- B.ZnCl2
- C.MgCl2
- D.Mg
Formula Unit Mass
Formula unit mass is the sum of the atomic masses of the atoms present in the formula unit of an ionic compound. It is calculated the same way as molecular mass but applied to ionic formula units.
Example 2: Calculate the formula unit mass of CaCl2.
Solution:
The atomic mass of Ca = 40 u.
The atomic mass of Cl = 35.5 u.
The formula unit mass of CaCl2 = atomic mass of Ca + 2 × atomic mass of Cl.
The formula unit mass of CaCl2 = 40 + 2 × 35.5 = 40 + 71 = 111 u.