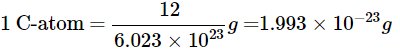Q1: Define the atomic mass unit.
Ans: One atomic mass unit is a mass unit equal to exactly one-twelfth (1/12th) the mass of one atom of carbon-12. Relative atomic masses of all elements are measured with respect to the carbon-12 standard.
According to IUPAC (International Union of Pure and Applied Chemistry), the atomic mass unit (written as u, the unified mass unit) is defined as the mass of one-twelfth of a carbon-12 atom.
1 amu = 1/12th mass of C612
Q2: Write down the formulae of
(a) Sodium oxide
Ans: Sodium oxide – Na2O
(b) Aluminium chloride
Ans: Aluminium chloride – AlCl3
(c) Sodium sulphide
Ans: Sodium sulphide – Na2S
(d) Magnesium hydroxide
Ans: Magnesium hydroxide – Mg(OH)2
Q3: Write down the names of compounds represented the following formulae:
(a) Al2(SO4)3
Ans: Al2(SO4)3 – Aluminium sulphate
(b) CaCl2
Ans: CaCl2 – Calcium chloride
(c) K2SO4
Ans: K2SO4 – Potassium sulphate
(d) KNO3
Ans: KNO3 – Potassium nitrate
(e) CaCO3
Ans: CaCO3 – Calcium carbonate
Q4: What is meant by the term chemical formula?
Ans: The chemical formula of a compound is a symbolic representation showing the types of atoms present and the number of each type in a single molecule (or formula unit) of that compound. It uses atomic symbols and numbers (subscripts) to indicate composition.
- They provide information on the elements that constitute the molecules of a compound and the ratio in which the atoms of those elements combine to form the molecules.
- Example: A water molecule contains two hydrogen atoms and one oxygen atom. Its chemical formula is H2O.
Q5: What are polyatomic ions? Give examples.
Ans: Polyatomic ions are groups of two or more atoms covalently bonded together that carry a net electrical charge and act as a single ion in chemical reactions. They behave as one charged unit.
Examples:
- Ammonium – NH4+
- Hydroxide – OH–
- Nitrate – NO3–
- Hydrogen carbonate – HCO3–
Q6: Write the chemical formulae of the following.
(a) Magnesium chloride
Ans: Magnesium chloride – MgCl2
(b) Calcium oxide
Ans: Calcium oxide – CaO
(c) Copper nitrate
Ans: Copper nitrate – Cu(NO3)2 (copper commonly exists as Cu2+ in this salt)
(d) Aluminium chloride
Ans: Aluminium chloride – AlCl3
(e) Calcium carbonate
Ans: Calcium carbonate – CaCO3
Q7: Give the names of the elements present in the following compounds.
(a) Quick lime
Ans: Quick lime – CaO
Elements present – Calcium, Oxygen
(b) Hydrogen bromide
Ans: Hydrogen bromide – HBr
Elements present – Hydrogen, Bromine
(c) Baking powder
Ans: Baking powder (sodium hydrogen carbonate+mild acid like tartaric acid) – NaHCO3
Elements present – Sodium, Hydrogen, Carbon, Oxygen
(d) Potassium sulphate
Ans: Potassium sulphate – K2SO4
Elements present – Potassium, Sulphur, Oxygen
Q8: What is the mass of –
Atomic mass of –
S = 32u, Al = 27u, Na = 23u, N = 14u, O = 16u
(a) 1 mole of nitrogen atoms?
Ans: Given the atomic mass of nitrogen is 14 u, 1 mole of nitrogen atoms has a mass of 14 g.
(b) 4 moles of aluminium atoms (Atomic mass of aluminium is 27)?
Ans: Mass of 1 mole of aluminium = 27 g. Therefore mass of 4 moles = 27 × 4 = 108 g.
Q9: Convert into mole.
Atomic mass of – C = 12u, H = 1u, O = 16u
(a) 12 g of oxygen gas
Ans: Molar mass of O2 = 16 × 2 = 32 g/mol
⇒ Number of moles = mass / molar mass
= 12 / 32 = 0.375 mol
(b) 20 g of water
Ans: Molar mass of H2O = (1 × 2) + 16 = 18 g/mol
⇒ Number of moles = mass / molar mass
⇒ Number of moles = 20 / 18 ≈ 1.11 mol
(c) 22 g of carbon dioxide
Ans: Molar mass of CO2 = 12 + (16 × 2) = 44 g/mol
⇒ Number of moles = mass / molar mass
⇒ Number of moles = 22 / 44 = 0.50 mol
Q10: State the Postulates of Dalton Theory?
Ans: Dalton’s atomic theory proposed that matter is made of small particles called atoms. Its main postulates are:
- All matter is composed of very small particles called atoms.
- Atoms are indivisible in chemical processes and cannot be created or destroyed in a chemical reaction.
- Atoms of a given element are identical in mass and chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in simple whole-number ratios to form compounds.
- A given compound always contains the same relative number and kinds of atoms (constant composition).
Q11: Find the percentage of water of crystallization in FeSO4.7H2O.
Ans: Atomic masses:
Fe = 55.9 u,
S = 32 u,
H = 1 u,
O = 16 u.
Molar mass of FeSO4·7H2O
= Fe + S + (O × 4) + 7 × (H2O)
= 55.9 + 32 + (16 × 4) + 7 × [(1 × 2) + 16 × 1]
= 55.9 + 32 + 64 + 7 × 18
= 151.9 + 126 = 277.9 g/mol
So, 1 of FeSO4 contains 126/277.6 g water of crystallization.
Mass of water of crystallization = 7 × 18 = 126 g per mole of the hydrated salt.
Percentage of water = (mass of water / molar mass of hydrated salt) × 100%
Thus, we get 126/277.6 x 100 = 0.4534 x 100 = 45.34%
The percentage of water of crystallization in FeSO4·7H2O is approximately 45.36%.
Q12: 2.42g of copper gave 3.025g of a black oxide of copper, 6.49g of a black oxide, on reduction with hydrogen, gave 5.192g of copper. Show that these figures are in accordance with the law of constant proportion?
Ans: Given:
Case A –
Mass of copper = 2.42 g
Mass of copper oxide = 3.025 g
Case B –
Mass of black copper oxide = 6.49 g
Mass of copper after reduction = 5.192 g
To verify the law of constant proportion, calculate the percentage of copper in the oxide in both cases.
Percentage of copper in Case A = (mass of copper / mass of copper oxide) × 100% = (2.42 / 3.025) × 100 = 80.00%
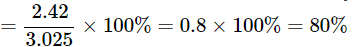
Percentage of copper in Case B = (mass of copper / mass of copper oxide) × 100% = (5.192 / 6.49) × 100 = 80.00%
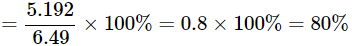
Both samples contain the same percentage of copper (80.00%). This shows that copper combines with oxygen in a constant proportion, confirming the law of constant proportions.
Q13: A compound was found to have the following percentage composition by mass Zn = 22.65%, S = 11.15% , H = 4.88% , O = 61.32% . The relative molecular mass is 287 g/mole . Find the molecular formula of the compound, assuming that all the hydrogen in the compound is present in water of crystallization.
Ans: Given percentages (per 100 g of compound):
Zn = 22.65 g,
S = 11.15 g,
H = 4.88 g
O = 61.32 g.
Atomic masses:
Zn = 65.4 u
S = 32 u
H = 1 u
O = 16 u.
⇒ Number of atoms
= percentage of element present in a compound × mass of compound / atomic mass × 100
Using the formula above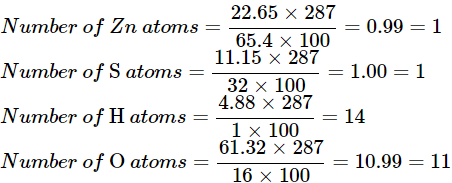
Empirical atom counts ≈ Zn1S1H14O11.
Assuming all hydrogen is in water of crystallization,
H14 corresponds to 7 molecules of H2O.
These 7 waters contribute 7 oxygen atoms,
leaving O atoms in the anhydrous part = 11 – 7 = 4.
Thus the formula for the anhydrous part is ZnSO4, with 7 H2O as waters of crystallization.
Therefore the compound is ZnSO4·7H2O.
Q14: Which element will be more reactive and why – the element whose atomic number is 10 or the one whose atomic number is 11?
Ans: The element with atomic number 11 is more reactive than the element with atomic number 10.
Reason: The element with atomic number 11 has electronic configuration (2, 8, 1). It has one electron in its outermost shell and can lose that electron easily to attain a stable noble-gas configuration, so it is more reactive (typical of alkali metals). The element with atomic number 10 has configuration (2, 8) and a full outer shell; it is already stable and thus much less reactive (a noble gas).
Q15: What are the failures of Dalton’s Atomic theory?
Ans: Dalton’s atomic theory was an important step, but it had several limitations:
- It did not account for subatomic particles: Atoms are not indivisible; electrons, protons and neutrons were later discovered.
- It did not account for isotopes: Atoms of the same element can have different mass numbers
Example: hydrogen ¹₁H, deuterium ²₁H , and tritium³₁H, have the same atomic number, but different mass numbers. - It did not account for isobars: Different elements can have atoms with the same mass number
Example: Ar4018 andCa4020, they have different atomic numbers, but the same mass number. - It assumed elements always combine in simple whole-number ratios; some complex molecules have larger whole-number ratios , though they still use whole numbers.
Example: sucrose C12H22O11 - It could not explain allotropy: Different forms of the same element (diamond and graphite for carbon) have different properties despite containing only one type of atom.
Q16: Calculate the Molecular Mass of
Atomic mass of – S = 32u, H = 1u, C = 12u, N = 14u, O =16u
(a) Ammonium sulphate (NH4)2SO4
Ans: Molar mass of (NH4)2SO4
= 2 × [N + (H × 4)] + S + (O × 4)
= 2 × [14 + (1 × 4)] + 32 + (16 × 4)
= 2 × 18 + 32 + 64 = 36 + 96 = 132 g/mol
(b) Penicillin C16H18N2SO4
Ans: Molar mass = (12 × 16) + (1 × 18) + (14 × 2) + 32 + (16 × 4)
= 192 + 18 + 28 + 32 + 64 = 334 g/mol
(c) Paracetamol C8H9NO2
Ans: Molar mass = (12 × 8) + (1 × 9) + 14 + (2×16)
= 96 + 9 + 14 + 32 = 151 g/mol
Q17: Write an experiment to show that cathode rays travel in a straight line?
Ans: An experiment to show that cathode rays travel in a straight line can be performed using a fluorescent coated discharge tube and a source of cathode rays, an opaque object, and a high voltage source.
Set-up and procedure:
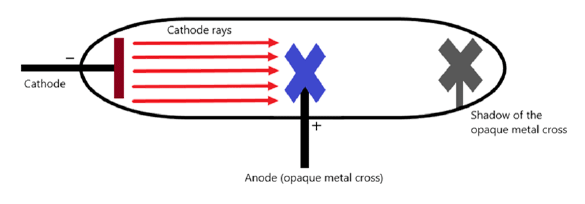
- Start the discharge so that cathode rays are produced inside the tube; the fluorescent coating will glow where rays strike.
- Place an opaque object in the path of the rays between the cathode and the fluorescent screen.
- When cathode rays strike against the screen, they produce fluorescence. But due to the placement of the opaque object, we will observe a sharp shadow being formed on the screen in the shape of the object.
- A sharp shadow of the object appears on the glowing screen behind it.
- A sharp shadow is produced only if the rays travel in straight lines; if the rays bent around the object no clear shadow would form.
This observation shows that cathode rays travel in straight lines.
Q18: What is radioactivity? What are the applications of radioisotopes?
Ans: Radioactivity is the spontaneous emission of radiation (alpha particles, beta particles or gamma rays)in the form of particles or high-energy photons from the nuclei of unstable atoms as they transform to more stable forms.
Applications of radioisotopes:
- Co-60 emits γ-radiation used in radiotherapy to treat cancer.
- I-131 is used in the diagnosis and treatment of thyroid disorders.
- P-32 is used in treating certain types of leukaemia and as a tracer in medical research.
- C-14 is used as a tracer in biochemical studies and for dating formerly living materials.
Q19: There are two elements C and B. C emits an α – particle and B emits a β – particle. How will the resultant elements change?
Ans: When an element emits an α particle, its atomic number decreases by 2 and its mass number decreases by 4 (an α particle is a He nucleus: 2 protons and 2 neutrons).
- So element C after α emission: atomic number → (Z – 2), mass number → (A – 4).
- When an element emits a β particle (electron), a neutron in the nucleus converts to a proton. Thus the atomic number increases by 1 while the mass number remains unchanged.
- So element B after β emission: atomic number → (Z + 1), mass number → A.
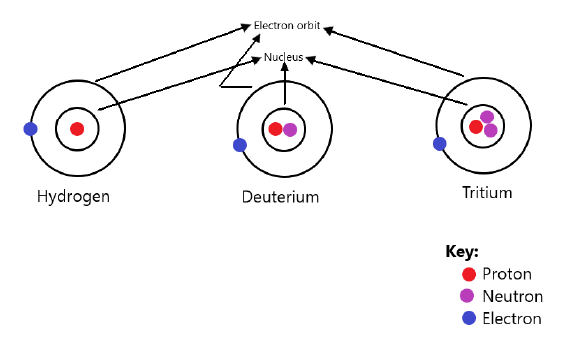
Q20: What are isotopes? Name the isotopes of hydrogen and draw the structure of their atoms.
Ans: Isotopes are atoms of the same element that have the same atomic number (same number of protons) but different mass numbers (different numbers of neutrons).
Example – Isotopes of hydrogen:
- Protium: ¹₁H (one proton, no neutron)
- Deuterium: ²₁H (one proton, one neutron)
- Tritium: ³₁H (one proton, two neutrons)
Structure of Isotopes of Hydrogen:
Q21: In a reaction, 5.3g of sodium carbonate reacted with 6g of ethanoic acid. The products were 2.2g of carbon dioxide, 0.9g water and 8.2g of sodium ethanoate. Show that these observations are in agreement with the Law of Conservation of Mass.
Sodium carbonate + Ethanoic acid → Sodium ethanoate + Carbondioxide + Water
Ans: The law of conservation of mass states mass is neither created nor destroyed in a chemical reaction.
So mass of reactants = mass of products.
Given:
Mass of sodium carbonate = 5.3 g
Mass of ethanoic acid = 6.0 g
Total mass of reactants = 5.3 + 6.0 = 11.3 g
Mass of products: sodium ethanoate = 8.2 g, CO2 = 2.2 g, H2O = 0.9 g
Total mass of products = 8.2 + 2.2 + 0.9 = 11.3 g
Mass of reactants = Mass of products = 11.3 g. This confirms the law of conservation of mass.
Q22: A 0.24g sample of compound of oxygen and boron was found by analysis to contain 0.096g of boron and 0.144g of oxygen. Calculate the percentage composition of the compound by weight.
Ans: Given:
Mass of sample = 0.24 g
Mass of boron = 0.096 g
Mass of oxygen = 0.144 g
Percentage of boron = (mass of boron / mass of sample) × 100%
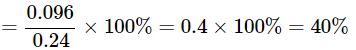
Percentage of oxygen = (mass of oxygen / mass of sample) × 100%
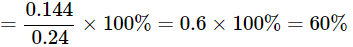
The compound contains 40% boron and 60% oxygen by mass.
Q23: If one mole of carbon atoms weighs 12 grams, what is the mass (in grams) of one atom of carbon?
Ans:
1 mole of carbon = 6.023 × 1023 atoms = 12 g
Mass of one carbon atom = 12 g / (6.023 × 1023) ≈ 1.993 × 10-23 g