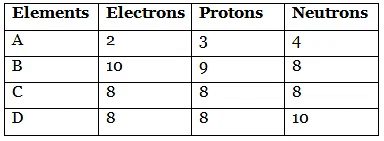
Q1: Number of electrons, protons and neutrons in chemical species A, B, C and D is given below:
Now, answer the following questions :
(a) What is the mass number of A and B?
(b) What is the atomic number of B?
(c) Which two elements represent a pair of isotopes and why?
(d) What is the valency of element C?
Also, justify your answers.
Ans:
(a) Mass number = Number of protons + Number of neutrons
Mass number of A = 3 + 4 = 7
Mass number of B = 9 + 8 = 17
(b) Atomic Number = Number of protons; therefore, atomic number of B = 9.
(c) Elements C and D represent a pair of isotopes.
Explanation: Isotopes are atoms of the same element that have the same atomic number (same number of protons) but different mass numbers (different numbers of neutrons). Since C and D have the same number of protons but different numbers of neutrons, they are isotopes of each other.
(d) To find the valency of element C, look at its electronic configuration.
Given that C has 8 electrons, its configuration is 2, 6.
It needs 2 more electrons to complete its octet and attain a stable noble gas configuration. Therefore, its valency = 2.
Q2: Describe in brief Rutherford’s alpha-particle scattering experiment with the help of a labelled diagram. Write any three important conclusions drawn from the experiment.
Ans: Rutherford’s alpha-particle scattering experiment:
Ernest Rutherford directed a beam of fast-moving alpha particles (positively charged) at a very thin gold foil and observed their scattering using a fluorescent screen. The alpha particles were produced by a radioactive source and the scattered particles produced tiny flashes of light on the screen, which were counted to record their paths.
Observations:
- Most alpha particles passed straight through the foil without deflection.
- Some alpha particles were deflected by small angles.
- A very few alpha particles (about 1 in 12,000) were deflected back, i.e., scattered through large angles.
Conclusions drawn:
- Most of the atom is empty space, because the majority of alpha particles passed through the foil without any deflection.
- There is a small, dense, positively charged nucleus at the centre of the atom; this concentrated positive charge repelled and deflected the positively charged alpha particles, causing the observed deflections.
- The size of the nucleus is very small compared to the overall size of the atom, since only a tiny fraction of alpha particles experienced large deflections.
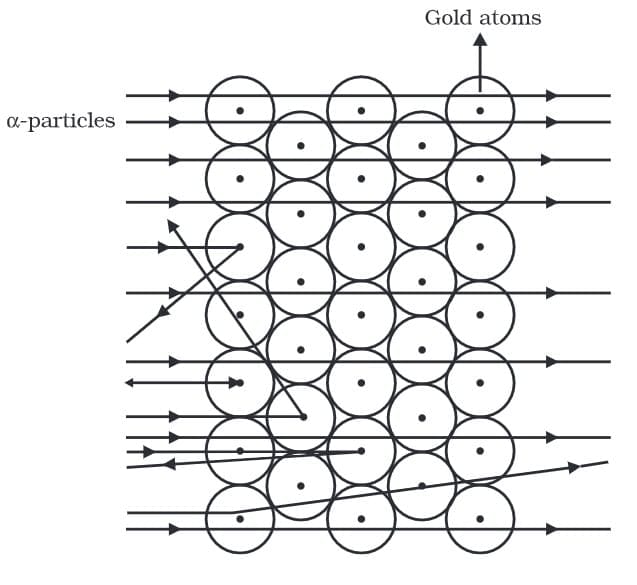 Scattering of alpha particles by a gold foil
Scattering of alpha particles by a gold foil
Q3: Give the number of electrons, protons and neutrons in 59CO27 and 108Ag47.
Ans : For 59Co27:
Atomic number = 27 → 27 protons
Number of electrons = 27 (since the atom is neutral)
Number of neutrons = Mass number − Atomic number = 59 − 27 = 32
For 108Ag47:
Atomic number = 47 → 47 protons
Number of electrons = 47 (neutral atom)
Number of neutrons = 108 − 47 = 61
Q4: Give the difference between isotopes and isobars.
Ans:
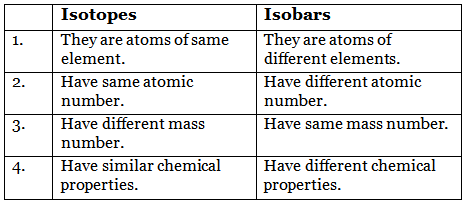
Difference between isotopes and isobars:
- Isotopes: Atoms of the same element that have the same atomic number (same number of protons) but different mass numbers (different numbers of neutrons). Example: 35Cl and 37Cl.
- Isobars: Atoms of different elements that have the same mass number but different atomic numbers (different numbers of protons). Example: 40Ca (Z = 20) and 40Ar (Z = 18) are isobars because both have mass number 40.
Q5: Chlorine occurs in nature in two isotopic forms with masses 35u and 37u in the ratio of 3 : 1. What should be the mass of a chlorine atom?
Ans: The average atomic mass of chlorine is calculated using the masses of its isotopes and their relative abundances.
Average atomic mass = (fractional abundance × mass of isotope) summed over isotopes.
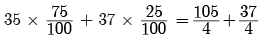
Using the given ratio 3 : 1, the fractional abundances are 3/4 and 1/4, respectively.
Average mass = (3 × 35u + 1 × 37u) / 4 = 142 / 4 = 35.5 u.
Hence, the average atomic mass of chlorine is 35.5 u.
Q6: An element 12X24 loses two electrons to form a cation, which combines with the anion of element 17Y35 formed by gaining an electron.
(i) Write the electronic configuration of element X.
(ii) Write the electronic configuration of the anion of element Y.
(iii) Write the formula for the compound formed by the combination of X and Y.
Ans:
(i) Atomic number of X = 12 → electronic configuration = 2, 8, 2.
On losing 2 electrons, it forms X²⁺ whose configuration becomes 2, 8 (like a noble gas).
(ii) Atomic number of Y = 17 → electronic configuration = 2, 8, 7.
On gaining 1 electron, Y forms the anion Y⁻ with configuration 2, 8, 8 (stable octet).
(iii) To balance charges: one X²⁺ ion will combine with two Y⁻ ions.
X²⁺ + 2 Y⁻ → XY₂.
So, the formula of the compound is XY₂.
Q7: Give reasons :
(i) The mass number of an atom excludes the mass of an electron.
(ii) The nucleus of an atom is charged.
(iii) Alpha-particle scattering experiment was possible by using a gold foil only and not by a foil of any other metal.
Ans:
(i) The mass of an electron is negligible compared to that of a proton or neutron (about 1/1836 of a proton). Therefore, the mass number, which counts the major contributors to atomic mass, includes only protons and neutrons and excludes electrons.
(ii) The nucleus contains protons, which are positively charged, while neutrons are neutral. As a result, the overall charge of the nucleus is positive.
(iii) Gold is highly malleable and can be hammered into extremely thin foils (a few atoms thick) without breaking. Such a thin foil was essential to allow alpha particles to pass through and to observe their scattering. This property made gold especially suitable for Rutherford’s experiment.
Q8: Give the postulates of Dalton’s atomic theory.
Ans: Dalton proposed an atomic theory in 1808 to explain the nature of matter. The main postulates of Dalton’s atomic theory are:
- All matter is composed of tiny indivisible particles called atoms.
- Atoms of a given element are identical in mass and properties.
- Atoms of different elements have different masses and properties.
- Atoms combine in simple whole-number ratios to form compounds.
- Atoms are indivisible and indestructible in chemical reactions (they are not created or destroyed in ordinary chemical changes).
- The relative number and kinds of atoms in a compound remain constant (fixed composition).
Note: Some parts of Dalton’s theory were later revised after the discovery of subatomic particles and isotopes, but his ideas laid the foundation of modern atomic theory.
Q9: Explain Bohr’s Model of the atom?
Ans: Bohr’s model was proposed to address limitations of Rutherford’s model. Its key postulates are:
- Quantised orbits: Electrons move in certain fixed circular orbits or energy levels around the nucleus without radiating energy. These orbits are labelled K, L, M, N or by principal quantum number n = 1, 2, 3, 4,…
- No radiation in stationary orbits: While an electron remains in a permitted orbit, it does not emit energy.
- Energy emission or absorption: An electron can move from one allowed orbit to another by absorbing energy (moving to a higher orbit) or emitting energy (moving to a lower orbit). The energy absorbed or emitted equals the difference between the two energy levels and is given by E = hν, where ν is the frequency of radiation.
- Quantised energy levels explain spectral lines: Since only specific energy differences are possible, atoms emit or absorb radiation of specific frequencies, producing discrete spectral lines.
Bohr’s model thus introduced the idea of quantised energy levels, explaining atomic spectra and the stability of atoms in a simple, conceptual way appropriate at the class 9 level.
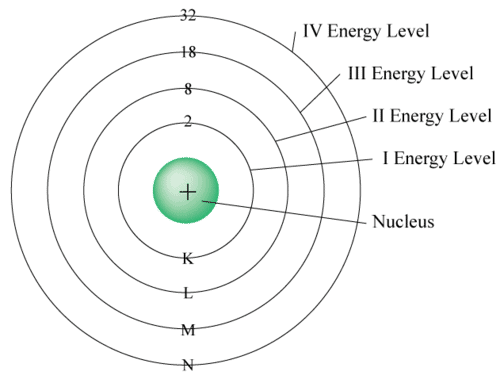
Q10: How are electrons disturbed in different shells or orbits?
Ans: Electron distribution in different shells:
The distribution of electrons in orbits (or energy levels) is explained by the following rules:
- Maximum electrons in a shell: The maximum number of electrons that can be accommodated in the nth shell is given by 2n².
- K-shell (n = 1): 2 × 1² = 2 electrons
- L-shell (n = 2): 2 × 2² = 8 electrons
- M-shell (n = 3): 2 × 3² = 18 electrons
- N-shell (n = 4): 2 × 4² = 32 electrons
- Maximum electrons in outermost shell: The outermost shell of an atom can have a maximum of 8 electrons (for the common main-group elements) to attain a stable configuration.
- Filling order of shells: Electrons occupy the inner shells first; an outer shell is not filled unless all inner shells are complete. Thus electrons are filled in a step-wise manner starting from the innermost shell.
Example: For sodium (atomic number 11), the electron distribution is 2, 8, 1, showing inner shells filled first and one electron in the outermost shell.