Multiple Choice Questions
Q1: The nucleus of an atom consists of ______.
(a) Protons
(b) Electrons and neutrons
(c) Protons and neutrons
(d) Neutrons
Ans: (c)
The nucleus contains protons (+) and neutrons (0); together they’re called nucleons.
Q2: What is the maximum number of electrons which can be present in K and L shells in an atom?
(a) 2 and 8
(b) 2 and 18
(c) 2 and 32
(d) 8 and 18
Ans: (a)
The K shell can hold a maximum of 2 electrons. The L shell can accommodate up to 8 electrons.
Q3: Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
Ans: (a)
Rutherford’s alpha-particle scattering experiment led to the discovery of the atomic nucleus. Key findings from the experiment include:
- Most alpha particles passed through the gold foil, indicating that atoms are mostly empty space.
- Only a few particles were deflected, suggesting that the positive charge of the atom is concentrated in a small area.
- A small fraction of particles rebounded, showing that the positive charge and mass are located in the nucleus.
This experiment fundamentally changed our understanding of atomic structure.
Q4: Isotopes of element have:
(a) The same physical properties
(b) Different chemical properties
(c) Different number of neutrons
(d) Different atomic numbers
Ans: (c)
Isotopes are atoms of the same element. They have the same atomic number but different mass numbers. This difference is due to varying numbers of neutrons.
Q5: Number of valence electrons in Cl– ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Ans: (b)
Cl– Ion has 18 electrons (17+1). Hence the number of valence electrons in Cl– ion is 8. Electronic distribution:
Cl – 2, 8, 7
Cl– – 2, 8, 8
Q6: An element with configuration 2,8,4 will tend to show valency
(a) 3
(b) 2
(c) 4
(d) 5
Ans: (c)
Electronic configuration 2,8,4 ⇒ outermost shell has 4 electrons.
To reach a stable octet, the atom needs 4 more (or would have to lose 4), so its valency = 4.
(Example: Silicon, Z = 14, commonly shows valency 4 by sharing electrons.)
Q7: Amongst element X (2,8,6) and Y (2,8,8) which is more reactive and why ?
(a) X because it is a metal
(b) Y because it is non metal
(c) X because it has 6 valence electrons
(d) Y because it is gas
Ans: (c)
Element X is more reactive due to its 6 valence electrons. This means it is more likely to gain 2 electrons to achieve a stable octet configuration.
- X has 6 valence electrons.
- It tends to gain 2 electrons.
- This helps it achieve a stable octet.
Q8: The nucleus of the hydrogen atom is called as
(a) Neutron
(b) Electron
(c) Proton
(d) Nucleons
Ans: (c)
The nucleus of a hydrogen atom contains a single proton.
Q9: Cathode rays get deflected in a electric field towards
(a) Positive plate
(b) Negative Plate
(c) No deflection takes place
(d) First towards negative plate and then towards positive plate
Ans: (a)
Cathode rays are negatively charged particles. They are attracted to the positive plate in an electric field. This attraction occurs because opposite charges attract each other.
Q10: The atomic number of an element ‘y’ is 20. The electronic configuration of the ion having inert gas configuration is
(a) 2,8,10
(b) 2,18
(c) 2,10,8
(d) 2,8,8
Ans: (d)
The ion with an inert gas configuration has lost 2 electrons. This results in an electronic configuration of 2, 8, 8.
Fill in the Blank
Q1: According to Bohr–Bury rules, the maximum number of electrons in the K-shell is ________ and in the L-shell is ________.
Ans: According to Bohr–Bury rules, the maximum number of electrons in the K-shell is 2 and in the L-shell is 8.
Q2: An atom is the smallest unit of an element which takes part in a _________.
Ans: An atom is the smallest unit of an element which takes part in a Chemical reaction.
Q3: Mass of an electron is 1/2000 times less than the mass of one atom of__________.
Ans: Mass of an electron is 1/2000 times less than the mass of one atom of hydrogen.
Q4: The K-shell of any atom cannot have more than _________ electrons.
Ans: The K-shell of any atom cannot have more than two electrons.
Q5: Isotopes are the atoms of ___________ element, having same atomic number but different mass number.
Ans: Isotopes are the atoms of the same element, having same atomic number but different mass number.
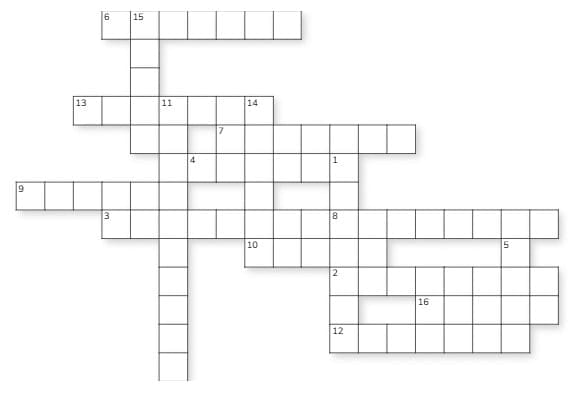
Crossword Puzzle

Ans: 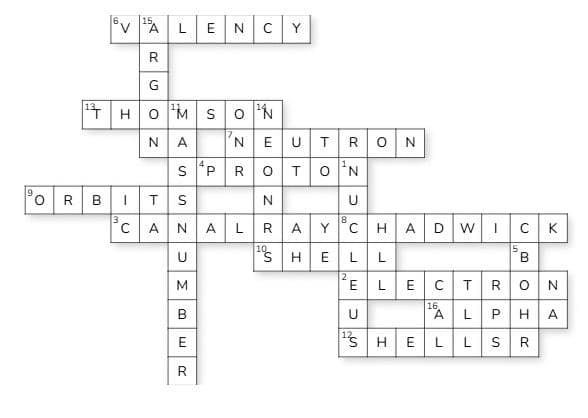
Very Short Answer Questions
Q1: Out of proton and neutron, which is heavier?
Ans: Neutron is slightly heavier (1.675 × 10–27 kg) than proton (1.67 × 10–27 kg).
Q2: Were neutrons known at the time Rutherford performed the scattering experiment?
Ans: No, neutrons were not known when Rutherford conducted his scattering experiment. They were discovered later by Chadwick in 1932. Rutherford’s experiment took place in 1911.
Q3: Who discovered canal rays and what do they indicate?
Ans: E. Goldstein discovered canal rays; they are positively charged radiations (led to proton concept).
Q4: What is the number of electrons in the valence shell of chlorine (Z = 17)?
Ans: 7 valence electrons (Cl: 2,8,7).
Q5. What is the basic difference between the isotopes of an element?
Ans: Isotopes have the same atomic number but different mass numbers (different neutrons).
Short Answer Questions
Q1: How will you find the valency of chlorine, sulphur and magnesium?
Ans: The valency of an element is determined by the number of valence electrons in its outermost shell. Here’s how to find the valency for chlorine, sulphur, and magnesium:
Chlorine (Cl):
- Atomic number: 17 (electron configuration: 2, 8, 7)
- Valence electrons: 7
- Needs 1 more electron to complete its octet (8).
- Valency: 1
Sulphur (S):
- Atomic number: 16 (electron configuration: 2, 8, 6)
- Valence electrons: 6
- Needs 2 more electrons to complete its octet (8).
- Valency: 2
Magnesium (Mg):
- Atomic number: 12 (electron configuration: 2, 8, 2)
- Valence electrons: 2
- It is easier for magnesium to lose its 2 valence electrons than to gain 6.
- Valency: 2
Q2: Describe Bohr’s model of the atom.
Ans: The special features of Bohr’s model are given below:
(1) An electron revolves in the orbit of atom with well-defined energy.
(2) Energy of orbits increases from inner shell to the outer shells i.e. energy for orbit nearest the nucleus is lowest.
(3) If energy is supplied then electron moves from lower orbit to the higher orbit and if an electron jumps from higher orbit (energy level) to the lower orbit (energy level) then energy is radiated as electromagnetic waves.
(4) Each orbit or shell represents an energy level. Such orbits are represented as K,L,M,N,O……….. and named from centre to outwards.
(5) The shell or orbits are associated with certain amount of energy and energy of orbits/shells increases from inward to outwards.eg K<L<M<N<O…………
Q3: What are the limitations of Rutherford’s model of the atom?
Ans: Limitations of Rutherford’s Model of the Atom
- The model does not explain the stability of the atom.
- According to the model, electrons orbiting the nucleus should emit energy due to acceleration.
- This energy loss would cause the electrons to spiral inward and eventually collide with the nucleus, leading to an unstable atom.
- However, we observe that atoms are generally quite stable.
Q4: Write the postulates of Bohr theory?
Ans: The postulates of Bohr’s theory are:
- Electrons move around the nucleus in specific circular paths known as orbits.
- Each orbit is linked to a fixed amount of energy.
- The larger the radius of the orbit, the greater the energy of the electrons.
- Electrons can transition between orbits by gaining or losing a specific amount of energy.
Q5: Explain, with examples, how atoms achieve an octet by losing, gaining, or sharing electrons.
Ans: By losing electrons (cations, metals):
- Atoms with 1–3 valence e⁻ lose them to get nearest noble-gas configuration.
- Examples: Na (2,8,1) → Na⁺ (2,8); Mg (2,8,2) → Mg²⁺ (2,8).
By gaining electrons (anions, non-metals):
- Atoms with 5–7 valence e⁻ gain electrons to complete 8.
- Examples: Cl (2,8,7) + e⁻ → Cl⁻ (2,8,8); O (2,6) + 2e⁻ → O²⁻ (2,8).
By sharing electrons (covalent bonds, non-metals):
- Atoms share pairs of e⁻ so each gets octet (H gets a duet).
- Examples: H₂ (duet for H), O₂ (double bond → octet), H₂O (O shares with 2H → octet), CH₄ (C shares 4 → octet).