Q1. What is a solution in science?
Answer: A solution is a uniform mixture in which the solute is evenly distributed in the solvent.
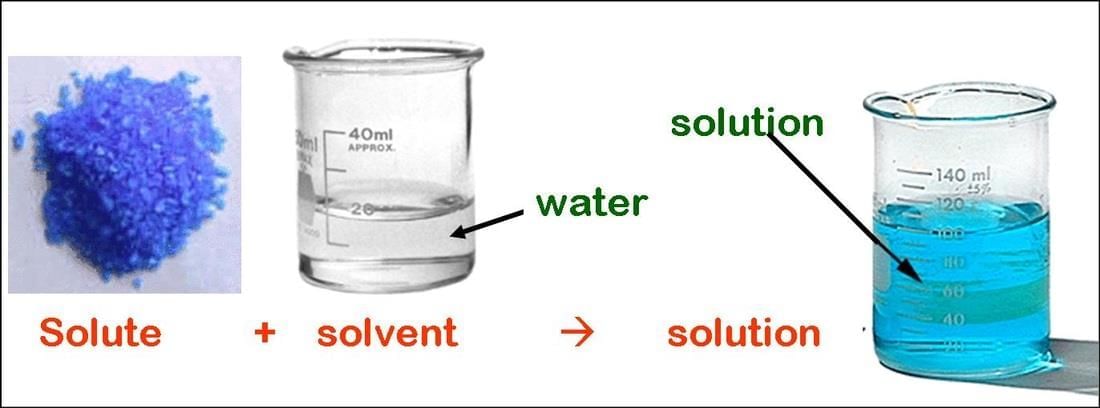
Q2. What is a solute in a solid–liquid solution?
Answer: A solute is the solid substance, like salt or sugar, that dissolves in a liquid.
Q3. What is a solvent in a solid–liquid solution?
Answer: A solvent is the liquid, like water, that dissolves the solute.
Q4. What is the basic formula for making a solution?
Answer: The basic formula is solute plus solvent equals solution.
Q5. Why does sugar in water form a clear mixture but sand does not?
Answer: Sugar dissolves to form a homogeneous solution, while sand does not dissolve and settles.
Q6. In air as a gaseous solution, which gas is the solvent?
Answer: In air, nitrogen is the solvent because it is present in the largest amount.
Q7. In vinegar, which component acts as the solute?
Answer: In vinegar, acetic acid is the solute and water is the solvent.
Q8. What does “saturated solution” mean?
Answer: A saturated solution has dissolved as much solute as possible at a given temperature.
Q9. What does “unsaturated solution” mean?
Answer: An unsaturated solution can still dissolve more solute at that temperature.
Q10. What is meant by concentration of a solution?
Answer: Concentration tells how much solute is present in a fixed amount of solution or solvent.
Q11. How does heating usually affect the solubility of most solids in liquids?
Answer: Heating usually increases the solubility of most solids in liquids.
Q12. How does temperature affect the solubility of gases in water?
Answer: The solubility of gases in water generally decreases as temperature increases.
Q13. Why is dissolved oxygen in water important?
Answer: Dissolved oxygen is vital because it allows aquatic plants and animals to breathe and live.
Q14. What is the definition of density?
Answer: Density is the mass per unit volume of a substance.
Q15. What is the formula for density?
Answer: The formula for density is Density = Mass / Volume.
Q16. Which has higher density: water at 4 °C or ice at 0 °C?
Answer: Water at 4 °C has higher density than ice at 0 °C.
Q17. Why does ice float on water?
Answer: Ice floats because it is less dense than liquid water.
Q18. What are the SI units of density?
Answer: The SI unit of density is kilogram per cubic metre (kg/m³).
Q19. Which units are commonly used for liquid density in labs?
Answer: Common lab units for liquid density are g/mL or g/cm³.
Q20. What instrument is used to measure mass in the lab?
Answer: A weighing balance is used to measure mass.
Q21. What instrument is used to measure liquid volume accurately?
Answer: A measuring cylinder is used to measure liquid volume accurately.
Q22. How should you read the water level in a measuring cylinder?
Answer: You should read the bottom of the meniscus at eye level.
Q23. How can you find the volume of an irregular solid like a stone?
Answer: You can find it by water displacement using a measuring cylinder.
Q24. What happens to density when a substance is heated?
Answer: On heating, volume usually increases and density generally decreases.
Q25. How does increasing pressure affect the density of a gas?
Answer: Increasing pressure compresses the gas, decreasing its volume and increasing its density.