Page No. 141
Q1: What do we get from cereals, pulses, fruits, and vegetables?
Ans: The things we get from cereals, pulses, fruits, and vegetables are as follows:
- Cereals are the source of carbohydrates and are the main source of energy.
- Pulses provide protein for growth and development.
- Vegetables and fruits are loaded with minerals, vitamins, carbohydrates, proteins and fats for overall development.

Page No. 142
Q1: How do biotic and abiotic factors affect crop production?
Ans: Two major factors that affect the crop are:
- Biotic factors like insects, rodents, pests, and many more spread the disease and reduce crop production.
- Abiotic factors like humidity, temperature, moisture, wind, rain, flood and many more destroy the crop raised.
Q2: What are the desirable agronomic characteristics for crop improvement?
Ans: The essential agronomic features required for crop improvement are:
- Profuse branching along with tallness in any fodder crop.
- Dwarfness in any cereals.
Page No. 143
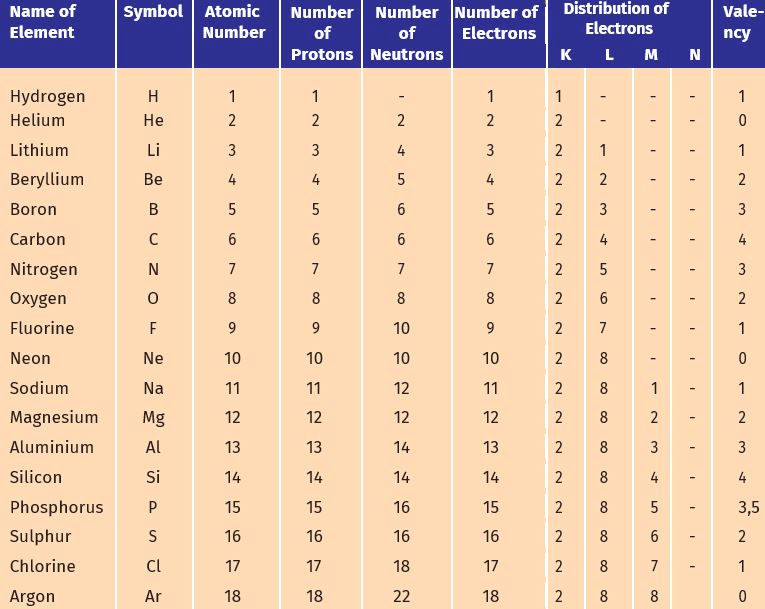
Q1: What are the macro-nutrients, and why are they called macro-nutrients?
Ans: Macro-nutrients are the fundamental elements that are used by plants in larger quantities. Macro-nutrients needed by the plants are
- Macro-nutrients as the constituents of protoplasm.
- Phosphorus, Nitrogen, and sulfur are present in proteins.
- Calcium exists in the cell wall.
- Magnesium is a significant component of chlorophyll.
Q2: How do plants get nutrients?
Ans: Plants get carbon from the air, oxygen from the air and water, and nutrients like nitrogen from the soil.
- Carbon: Plants take in carbon dioxide (CO₂) from the air through tiny holes in their leaves and use sunlight to make food (photosynthesis).
- Oxygen: They absorb oxygen from the air for respiration and also get it from water through their roots.
- Nutrients: Roots absorb essential nutrients like nitrogen, phosphorus, and potassium from the soil to help the plant grow strong and healthy.
Page No. 144
Q1: Compare the use of manure and fertilisers in maintaining soil fertility.
Ans:
- Manure improves soil quality with added nutrients.
- Manure provides extra organic matter called humus to the soil, therefore increasing the water retention capacity of sandy soils and drainage in clayey soil.
- Manures reduce soil erosion.
- They provide food for soil-friendly bacteria, which are helpful in growing crops.
The effects of fertilisers are
- Fertilisers make the soil too dry and powdery and raise the rate of soil erosion.
- The organic matter decreases by decreasing the porosity of the soil; hence, the plant roots do not get enough oxygen.
- The nature of soil changes, either basic or acidic.
Page No. 145
Q1: Which of the following conditions will give the most benefits? Why?
(a) Farmers use high-quality seeds, do not adopt irrigation or use fertilisers.
(b) Farmers use ordinary seeds, adopt irrigation, use fertilisers and use crop protection measures.
(c) Farmers use quality seeds, adopt irrigation, use fertiliser and use crop protection measures.
Ans: Option (c) will give the most benefits because the use of good quality seeds is not only sufficient until the soil is properly irrigated, enriched with fertilisers and protected from biotic factors.
Page No. 146
Q1: Why should preventive measures and biological control methods be preferred for protecting crops?
Ans: Over-exposure to chemicals leads to environmental problems; hence, biological methods are preferred for protecting crops from pathogens, insects and rodents, along with increasing production. Since chemicals are harmful to plants and also to the animals that feed on them, bio-pesticides are used as a safe way of crop protection.
Q2: What factors may be responsible for the losses of grains during storage?
Ans: Biotic and Abiotic factors are responsible for the loss of grains during storage like
- Rodents
- Pests
- Insects
- Fungi
- Bacteria
- Sunlight
- Flood
- Rain
- Temperature
- Moisture
Page No. 147
Q1: Which method is commonly used for improving cattle breeds and why? How is cross-breeding useful in animals?
Ans: To improve the cattle breeds, we generally use the cross-breeding method. It is a process in which a cross is made between indigenous varieties of cattle by exotic breeds to get a crossbreed that is high-yielding. During cross-breeding, the desired characters taken into consideration are that the offspring should be high-yielding, should have early maturity and should be resistant to diseases and climatic conditions.
Page No. 148
Q1: Discuss the implications of the following statement. It is interesting to note that poultry is India’s most efficient converter of low-fibre foodstuff (which is unfit for human consumption) into highly nutritious animal protein food.
Ans: Poultry farming aims to raise domestic birds for egg and chicken meat purposes. These domestic birds feed on animal feeds which mainly consist of roughages for getting good quality feathers, eggs, chicken and nutrient-rich manure. For these reasons, it is said that “poultry is India’s most efficient converter of low-fibre foodstuff into highly nutritious animal protein food.”
Q2: What management practices are common in dairy and poultry farming?
Ans:
- Well-designed Hygienic shelter for dairy animals and poultry birds.
- Good quality, proper food and fodder are provided to dairy animals and poultry birds.
- Importance for animal health by prevention and cure of diseases caused by bacteria, viruses, or fungi.
- Sunlight-feasible and air,y ventilated shelter for animals.
Q3: What are the differences between broilers and layers and in their management?
Ans:
Broilers
- The poultry bird raised for meat purposes is called a broiler. Broilers feed on protein-rich, adequate-fat food. The level of vitamins A and K is kept high in poultry feeds.
Layers
- The egg-laying poultry bird is called a layer. The housing, environmental and nutritional requirements of broilers vary from those of egg layers. Layers require proper lighting and enough space.
Page No. 150
Q1: How are fish obtained?
Ans: Fishes are obtained in two ways:
- Capture fishing: Obtaining fish from natural resources.
- Culture Fishery: Culturing of fish in freshwater ecosystems, like rivers, ponds and lakes, also including marine.
Q2: What is the advantage of composite fish culture?
Ans: The advantages of composite fish culture are:
- In a single fish pond, a combination of 5 or 6 types of fish species can be cultured since they do not compete for food among themselves
- Food resources can be completely utilised
- Survival of the fish also increases
- More yield
Q3: What are the desirable characters of the varieties suitable for honey production?
Ans: Desirable characters in varieties for honey production are:
- The variety of bees should yield a large amount of honey.
- The bees should stay for a longer period in the beehives.
- The bees should not sting much.
- Bees should be disease-resistant.

Honey Production
Q4: What is pasturage and how is it related to honey production?
Ans: Pasturage refers to the availability of flowers to the bees for easy accessibility for pollen collection and nectar. The kinds of flowers available will determine the taste of the honey; hence, Pasturage is the main reason for good-quality honey.
Page No. 151
Exercises
Q1: Explain any one method of crop production which ensures high yield.
Ans: Plant breeding is one of the methods adopted for high-yield plant breeding and is implemented to improve the varieties of crops by breeding plants. Plants from various places/areas are picked up with preferred traits, and then the process of hybridisation or cross-breeding is done among these diversities to get a crop/plant of anticipated characteristics.
Q2: Why are manure and fertilisers used in fields?
Ans: Manures and fertilisers are used to enrich the soil quality and improve the yield. They also help in controlling diseases. Manure and fertilisers replenish the soil by supplying nutrients to the soil. They are excellent sources of potassium, phosphorus and nitrogen, which assist in the healthy development of plants. Manures and fertilisers mainly improve the fertility of the soil.
Q3: What are the advantages of inter-cropping and crop rotation?
Ans: Inter-cropping
- Checks pests and rodents, and hence decreases the chances of the spoiling of whole crops
- Decreased chances of soil erosion
- Reduced loss of crops with high-yield
- Less water requirement
Crop rotation
- Farmers can grow two or three crops annually
- Pulses take nitrogen directly from the atmosphere and hence require a minimal amount of fertilisers
- Both fruits and vegetables can be grown easily
- Best use of land with a proper supply of nutrients
Q4: What is genetic manipulation? How is it useful in agricultural practices?
Ans:
- Genetic manipulation is a process in which the transfer of genes takes place from one organism to another. Here, a gene of a particular character is introduced into the chromosome cell, resulting in a transgenic plant.
- Example: Bt Cotton is a genetically modified crop that carries bacterial genes that protect this plant from insects. These are used in plants like brinjal, cabbage, rice, cauliflower, and maize crops to get protection from insects.
Q5: How do storage grain losses occur?
Ans: Storage grain losses occur due to various abiotic and biotic factors.
Abiotic factors
- Humidity
- Air
- Temperature
- Flood
- Wind
Biotic factors
- Insects
- Rodents
- Pesticides
- Bacteria
- Mites
- Birds
Q6: How do good animal husbandry practices benefit farmers?
Ans: Good practice of animal husbandry benefits farmers in the following ways:
- Yields in good-quality cattle
- Better quality of milk production
- Use in agriculture for carting, irrigation and tilling
Q7: What are the benefits of cattle farming?
Ans: The benefits of cattle farming are
- Cattle are used for agricultural purposes
- Generation of good-quality cattle
- Milking and meat purpose
- The skin of cattle is used for the leather and wool industry
Q8: For increasing production, what is common in poultry, fisheries, and beekeeping?
Ans: For increasing production, cross-breeding techniques are adopted in poultry, fisheries and bee-keeping. Along with these techniques, regular and proper maintenance methods are useful in improving production.
Q9: How do you differentiate between capture fishing, mariculture, and aquaculture?
Ans: Differences between capture fishing, mariculture, and aquaculture
Fishing
- Fish are obtained from natural resources, like ponds, canals, rivers,
- Locating fish is easy and can be captured by using fishing nets.

Fishing
Mariculture
- A method of marine fish culture in the open sea.
- Fish can be located with the help of satellites and echo sounders. These can be caught by many kinds of fishing nets using fishing boats.

Mariculture
Aquaculture
- Production of fish from freshwater and brackish water resources.
- It can be located easily and caught using fishing nets.

Aquaculture
 Windmills harnessing the Kinetic energy
Windmills harnessing the Kinetic energy
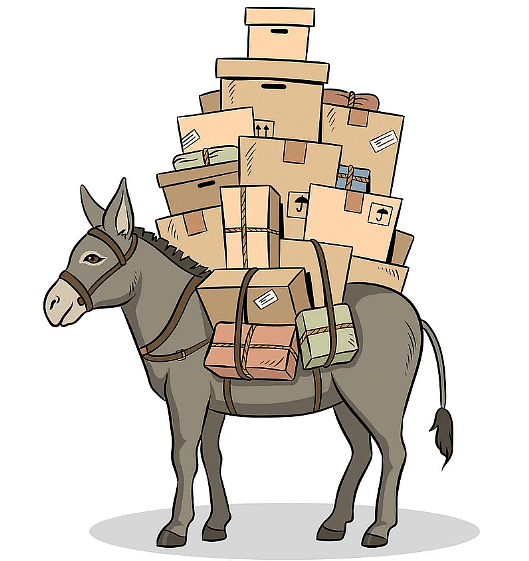



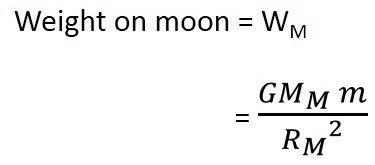
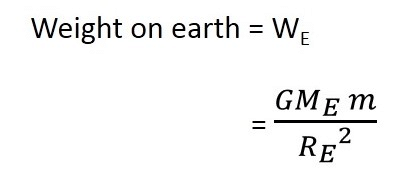

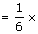
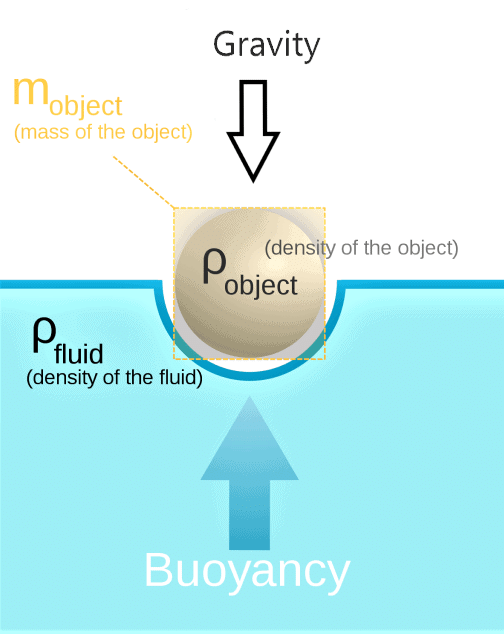
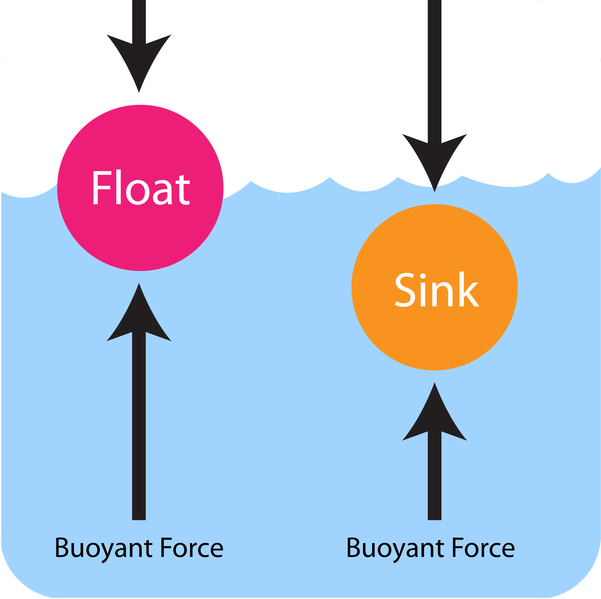
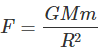
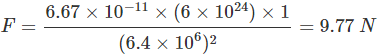
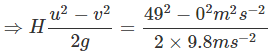
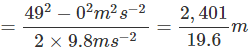
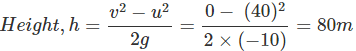
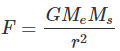
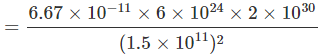
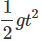
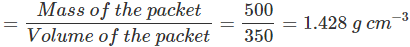
 ). Hence, it will sink in water.
). Hence, it will sink in water.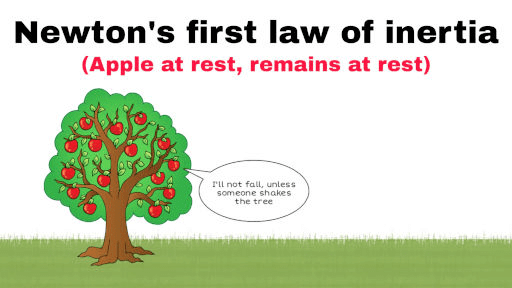
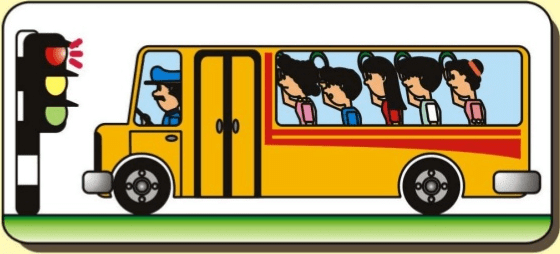 The bus stops suddenly, Passenger jerks forward
The bus stops suddenly, Passenger jerks forward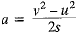
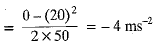
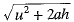
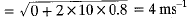



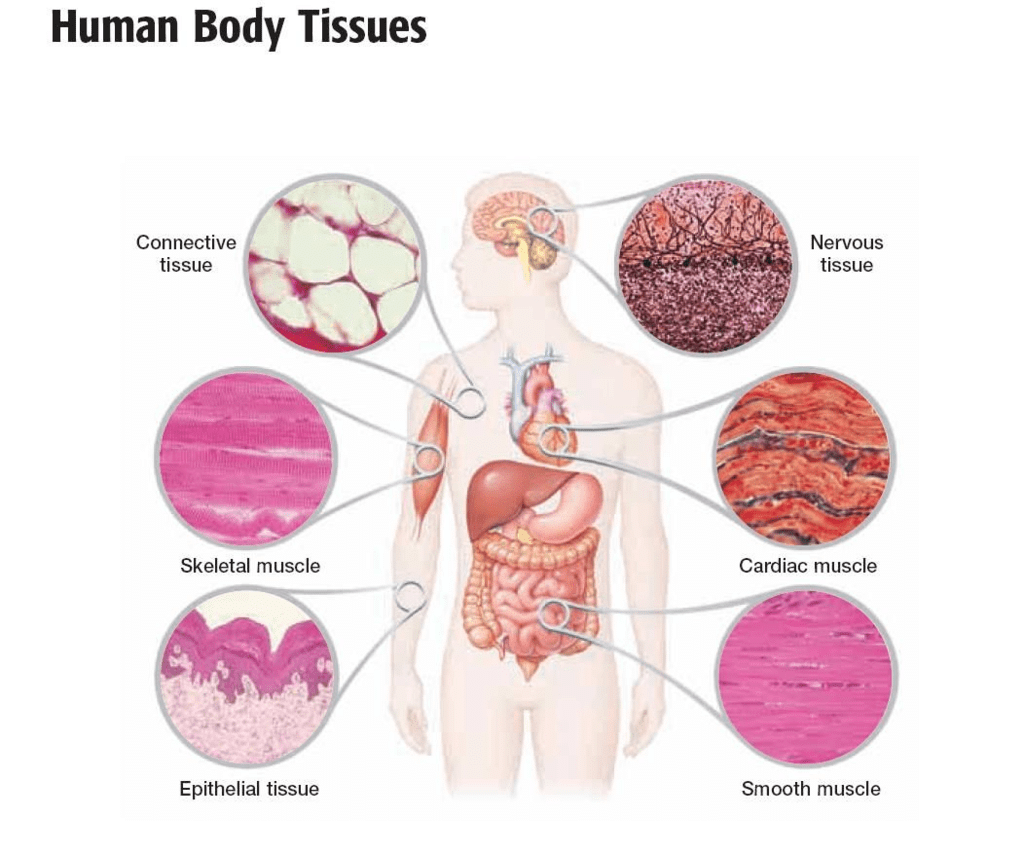
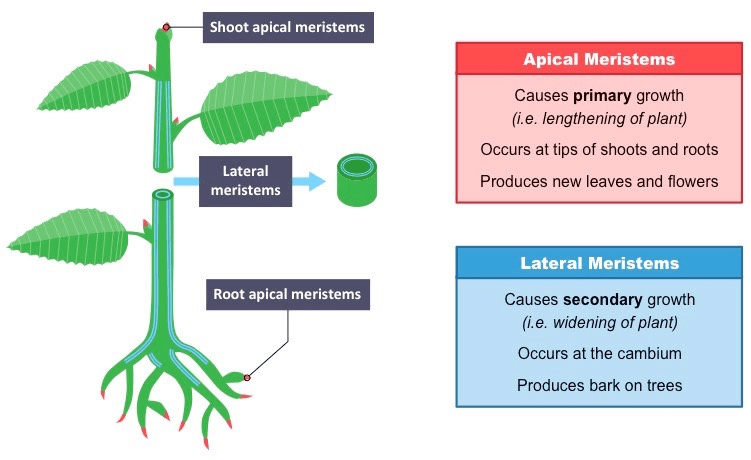
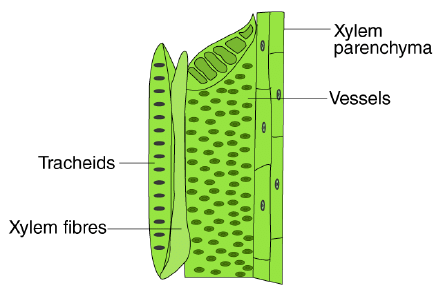
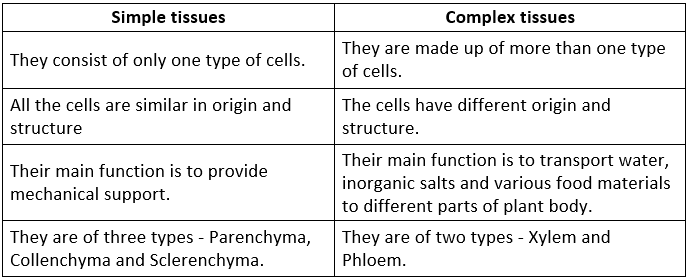

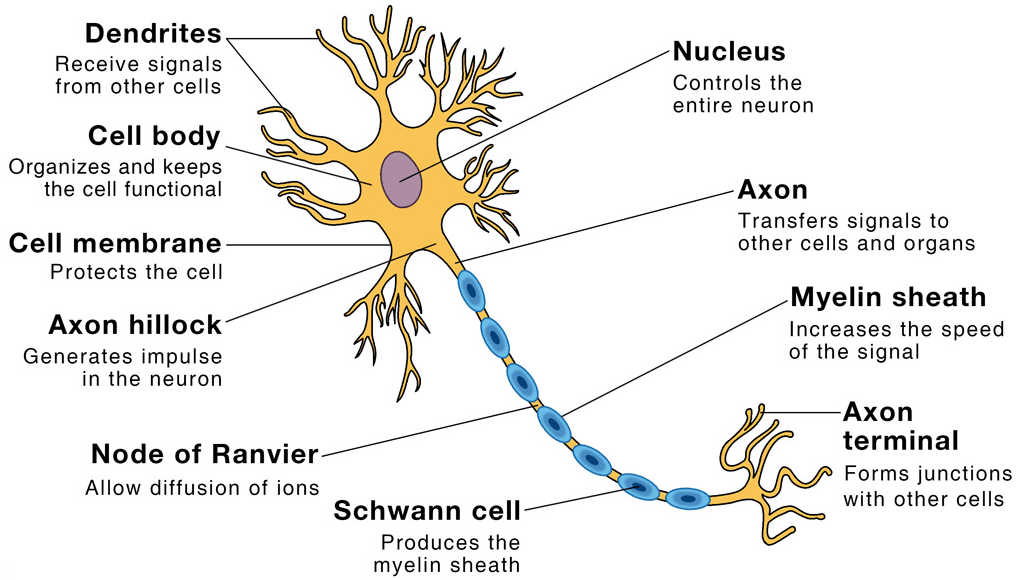
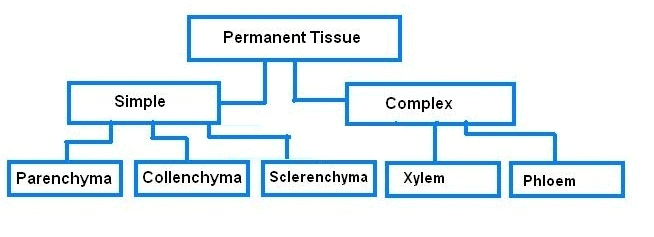
 Microscope and Cork cells
Microscope and Cork cells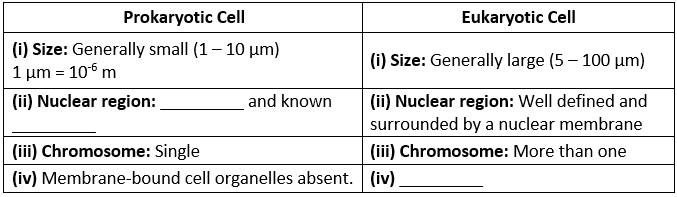
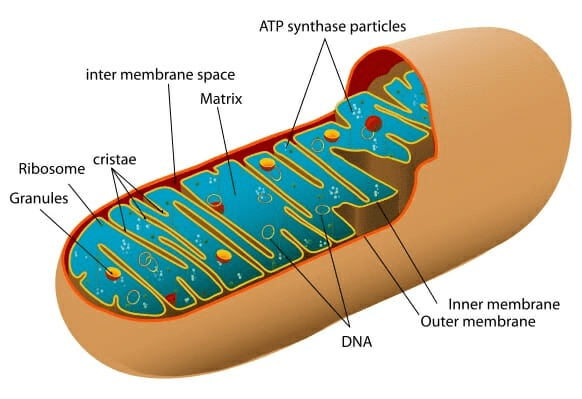 Mitochondria
Mitochondria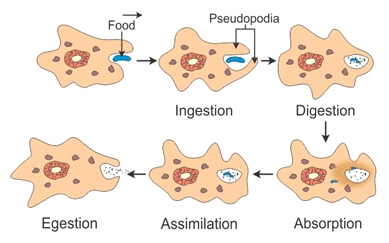 Nutrition in Amoeba
Nutrition in Amoeba