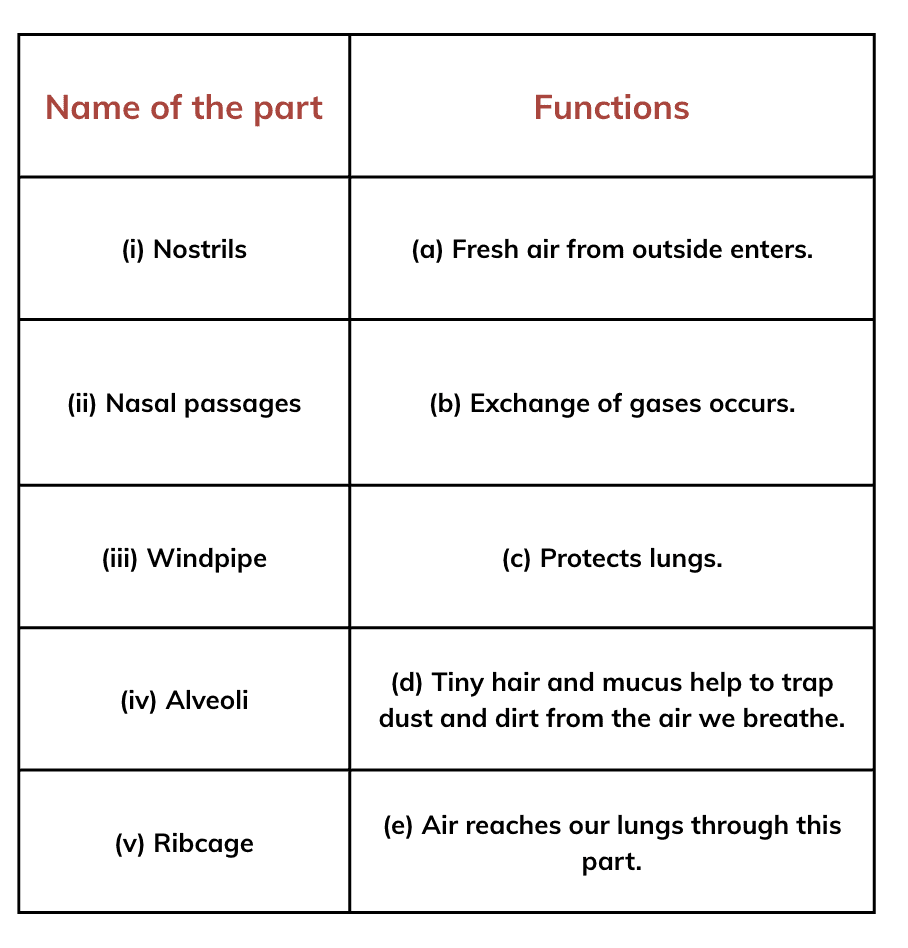
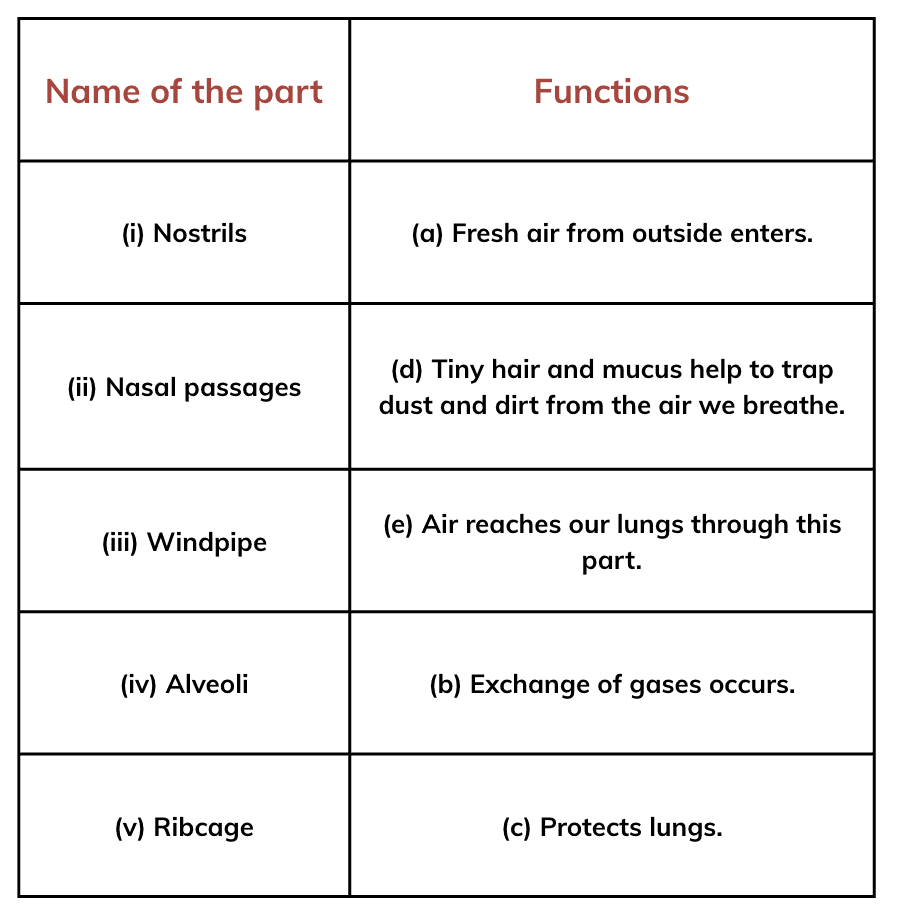
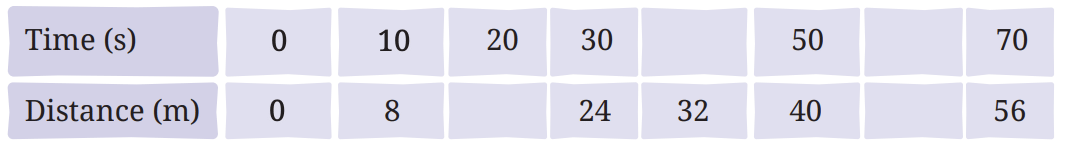
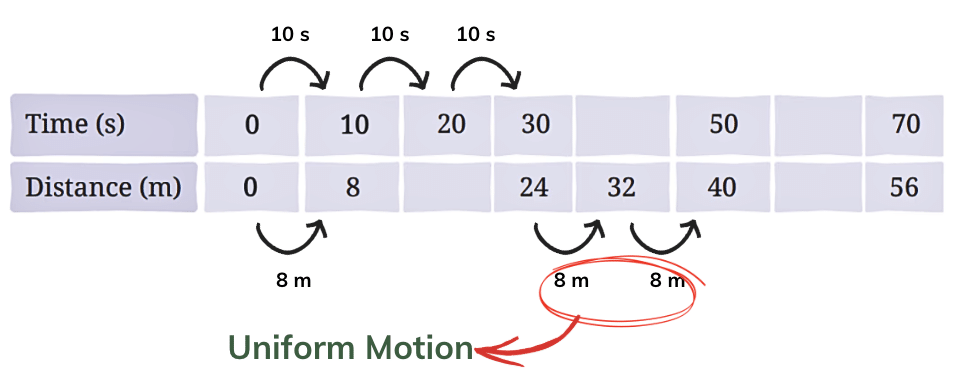
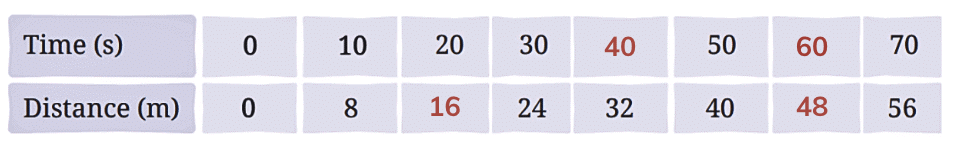
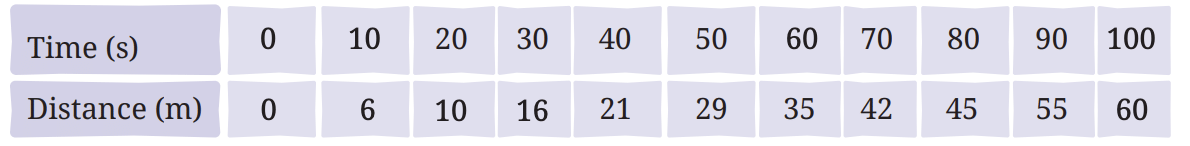
Q1: Complete the following table.
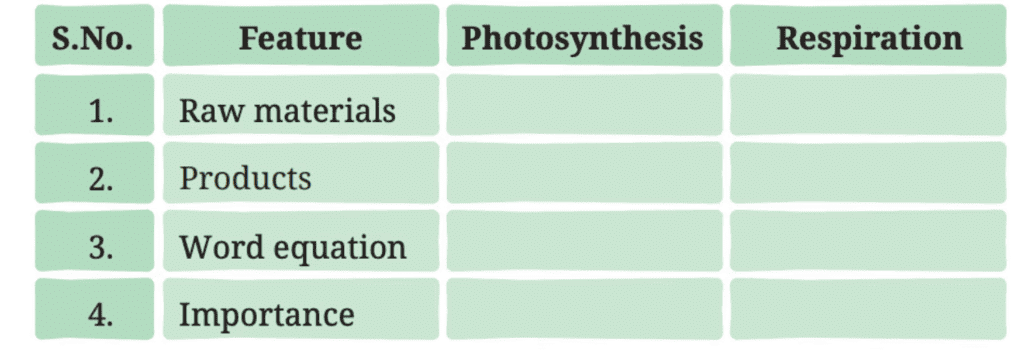
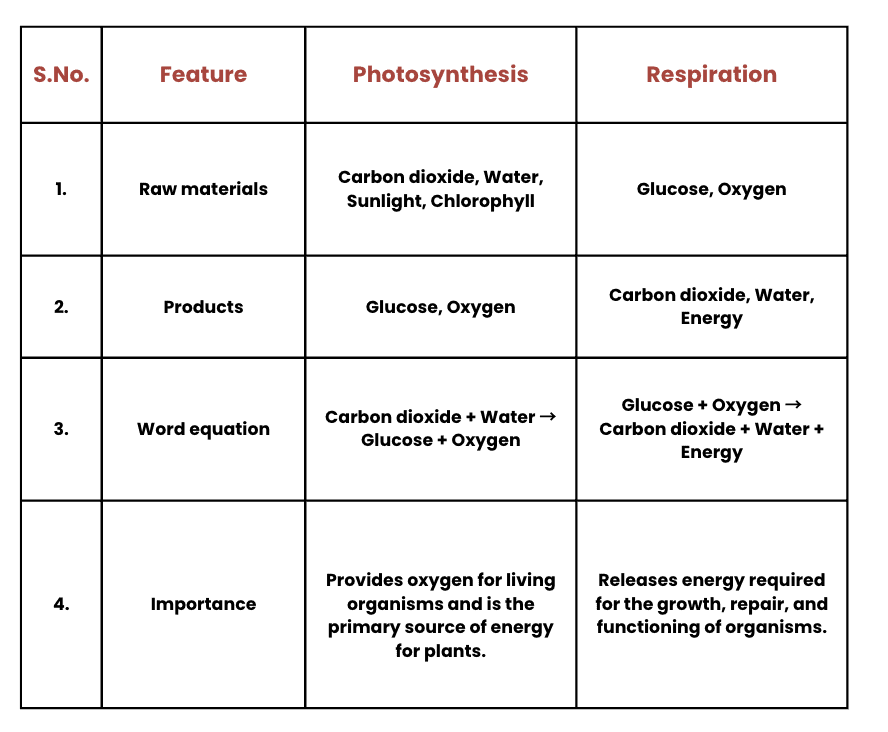
Ans:
Q2: Imagine a situation where all the organisms that carry out photosynthesis on the earth have disappeared. What would be the impact of this on living organisms?
Ans: If all photosynthetic organisms disappeared, there would be no source of oxygen production or food for herbivores. This would disrupt the entire food chain, leading to the collapse of ecosystems, and animals would eventually die due to lack of oxygen and food.
- No oxygen production: Plants give us oxygen through photosynthesis. Without them, there would be less oxygen in the air, making it hard for humans and animals to breathe.
- More carbon dioxide: Photosynthesis helps take in carbon dioxide from the air. Without it, the carbon dioxide levels would rise, causing the Earth to become warmer and making the climate unstable.
- Loss of food supply: Plants are the primary source of food for herbivores, and herbivores are eaten by carnivores. If photosynthesizing organisms disappeared, the entire food chain would collapse. Without plants to produce food (glucose), animals would starve, and many species would not survive.
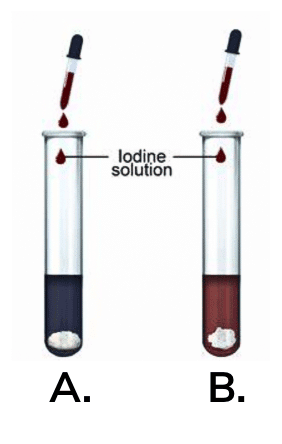
Q3: . Where does the starch in potatoes come from? Where is the food synthesised in the plant, and how does it reach the potato?
Ans: The starch in potatoes comes from the food made in the leaves of the plant. The plant makes food in the form of glucose through a process called photosynthesis, which happens in the leaves when the plant takes in sunlight, carbon dioxide, and water.
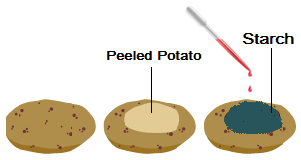
Once the leaves make glucose, it travels through the plant to other parts, like the potato, through special tubes called phloem. The potato acts like a storage place where the glucose is turned into starch and stored for later use, which is why when you test a potato with iodine, you see a blue-black color, showing the presence of starch.
Q4: Does the broad and flat structure of leaves make plants more efficient for photosynthesis? Justify your answer.
Ans: Yes, the broad and flat structure of leaves make plants more efficient for photosynthesis. This is because of following reasons:
- The broad shape also helps the leaf to face the Sun directly, maximizing the amount of light it receives throughout the day.
- The broad and flat shape of leaves provides a larger surface area, allowing more sunlight to be captured. The more sunlight the leaf can absorb, the more energy it can use to produce food through photosynthesis.
- The flat shape helps the leaf to spread out and have more stomata (small pores) on its surface. These stomata allow the plant to take in carbon dioxide (which is needed for photosynthesis) and release oxygen more efficiently.
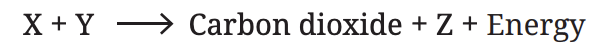
Q5: X is broken down using Y to release carbon dioxide, Z, and energy.
X, Y, and Z are three different components of the process. What do X, Y, and Z stand for?
Ans:
- X: Glucose – Glucose is the sugar that is broken down in the process.
- Y: Oxygen – Oxygen is used to help break down glucose in respiration.
- Z: Water – Water is a product of the respiration process.
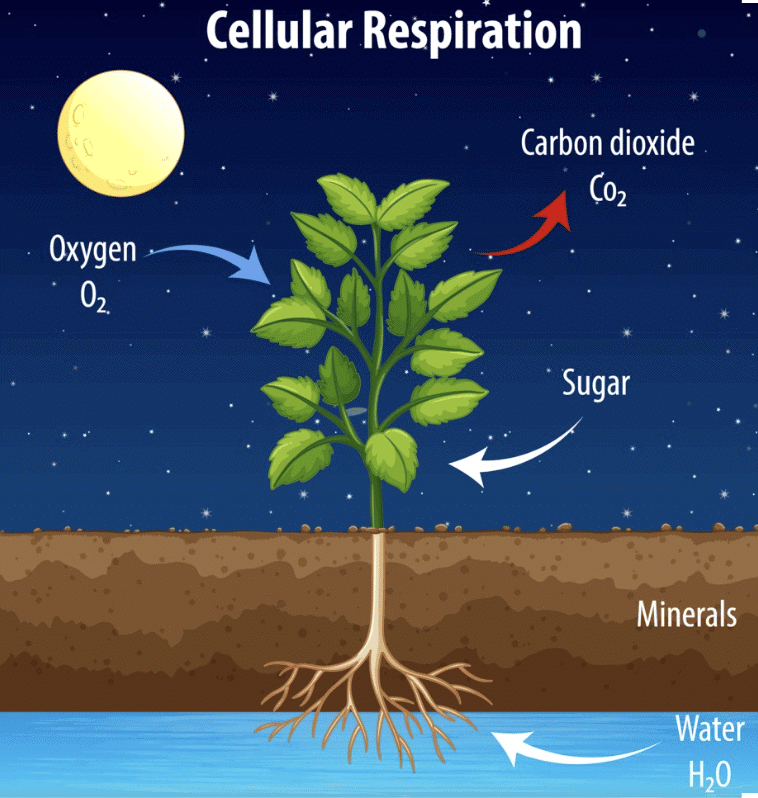
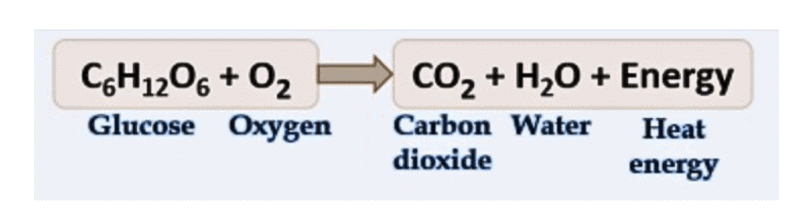
Thus, the equation represents the process of respiration in plants:
Glucose + Oxygen → Carbon dioxide + Water + Energy
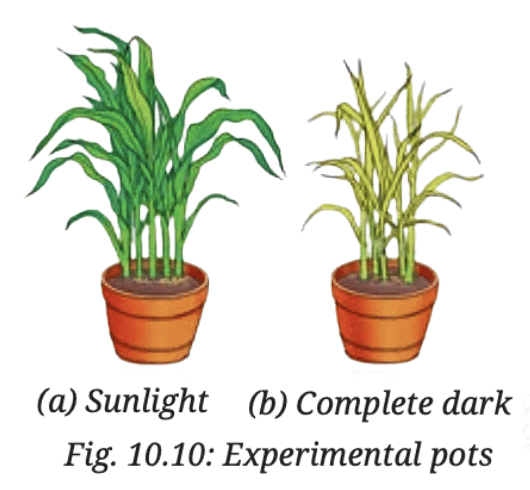

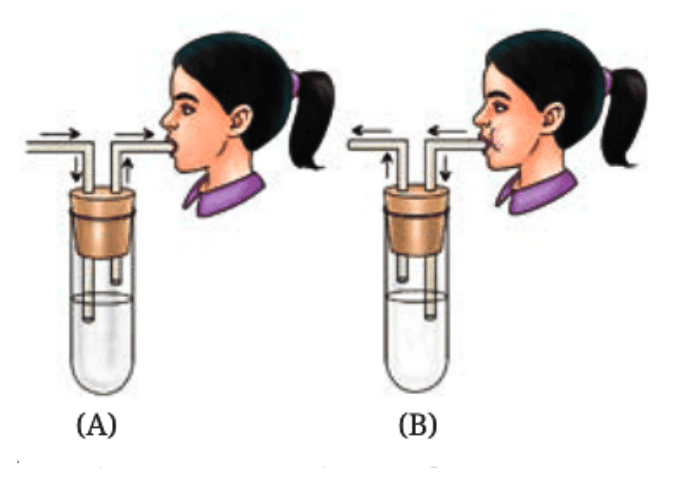
Q6: Krishna set-up an experiment with two potted plants of same size and placed one of them in sunlight and the other in a dark room, as shown in Fig. 10.10.Answer the following questions:
(i) What idea might she be testing through this experiment?
Ans: Krishna is testing the effect of sunlight on plant growth and starch production in plants.
(ii) What are the visible differences in plants in both the conditions?
Ans: The plant in sunlight will show better growth, with green leaves and starch production. This is because sunlight is required for photosynthesis, and the plant will produce starch as a result.
The plant in the dark will show stunted growth, with smaller or yellowed leaves, and no starch production. Without light, the plant can’t do photosynthesis, so it won’t be able to produce starch.
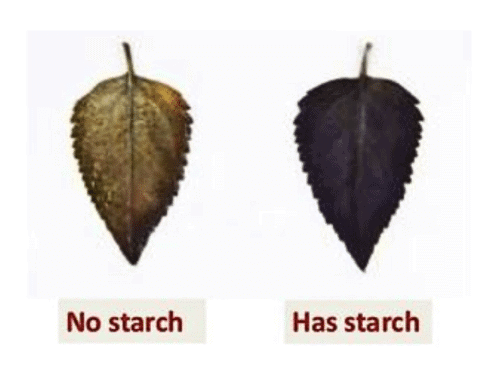
(iii) According to you, leaves of which plants confirm the iodine test for the presence of starch?
Ans: The leaves of the plant kept in sunlight will show a positive iodine test, indicating the presence of starch. The iodine will turn blue-black when it reacts with starch. Since sunlight is required for photosynthesis, the plant in sunlight will have made starch, while the plant in the dark will not have any starch to react with iodine.
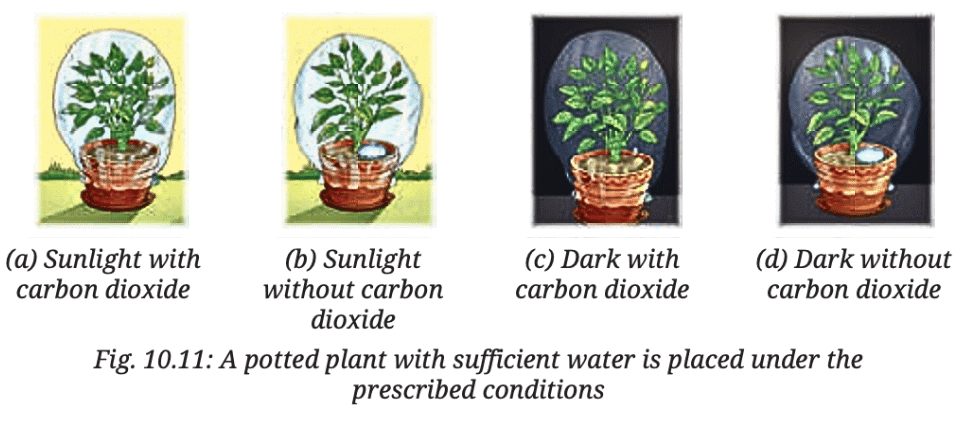
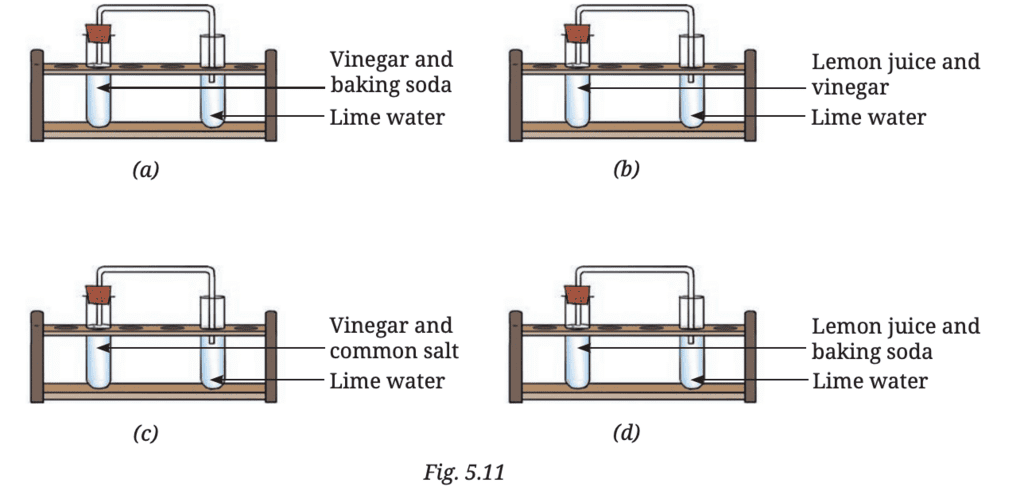
Q7: Vani believes that ‘carbon dioxide is essential for photosynthesis’. She puts an experimental set-up, as shown in Fig. 10.11, to collect evidence to support or reject her idea.Answer the following questions:
(i) In which plant(s) in the above set-up(s) will starch be formed?
Ans: Starch will be formed in the plant under (a) Sunlight with carbon dioxide because sunlight and carbon dioxide are both essential for photosynthesis. In this condition, the plant can perform photosynthesis and produce starch.
(ii) In which plant(s) in the above set-up(s) will starch not be formed?
Ans: Starch will not be formed in the plants under (b) Sunlight without carbon dioxide, (c) Dark with carbon dioxide, and (d) Dark without carbon dioxide.
- In (b), there’s no carbon dioxide, which is required for photosynthesis.
- In (c), though there is carbon dioxide, there’s no sunlight, which is needed for photosynthesis.
- In (d), there’s neither sunlight nor carbon dioxide, so photosynthesis cannot happen.
(iii) In which plant(s) in the above set-up(s) will oxygen be generated?
Ans: Oxygen will be generated in the plants under (a) Sunlight with carbon dioxide because both sunlight and carbon dioxide are essential for photosynthesis, during which oxygen is produced.
(iv) In which plant(s) in the above set-up(s) will oxygen not be generated?
Ans: Oxygen will not be generated in the plants under (b) Sunlight without carbon dioxide, (c) Dark with carbon dioxide, and (d) Dark without carbon dioxide.
- In (b), there’s no carbon dioxide, so photosynthesis cannot occur, and oxygen is not produced.
- In (c), there’s no sunlight, so photosynthesis cannot occur, and oxygen is not produced.
- In (d), there’s no sunlight or carbon dioxide, so oxygen is not produced.
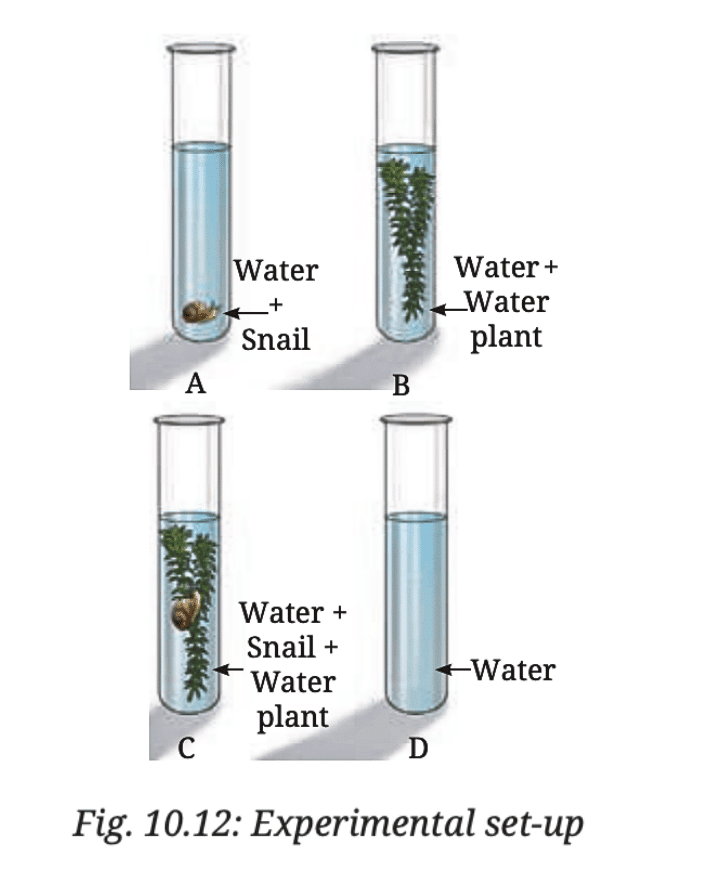
Q8: Ananya took four test tubes and filled three-fourth of each test tube with water. She labelled them A, B, C, and D (Fig. 10.12). In test tube A, she kept a snail; in test tube B, she kept a water plant; in test tube C, she kept both a snail and a plant. In test tube D, she kept only water. Ananya added a carbon dioxide indicator to all the test tubes. She recorded the initial colour of water and observed if there are any colour changes in the test tubes after 2–3 hours. What do you think she wants to find out? How will she know if she is correct?
Ans: Ananya is likely conducting an experiment to investigate the role of carbon dioxide (CO₂) in the process of respiration and photosynthesis in aquatic organisms. She is testing whether the presence of a snail (which respires) and/or a water plant (which carries out photosynthesis) affects the level of carbon dioxide in the water over time.
Activity Explanation:
- Test tube A (with a snail): The snail respires and releases carbon dioxide (CO₂). If the carbon dioxide indicator turns a different color (e.g., from yellow to green), it will indicate an increase in CO₂ levels in the water due to respiration.
- Test tube B (with a water plant): The water plant carries out photosynthesis, using carbon dioxide and releasing oxygen. If the indicator shows a decrease in CO₂ (e.g., from yellow to blue), it will indicate that the plant is taking in carbon dioxide during photosynthesis.
- Test tube C (with both a snail and a water plant): In this setup, both respiration (from the snail) and photosynthesis (from the plant) will occur. The snail will produce carbon dioxide, and the plant will consume it for photosynthesis. If there’s no change in color, it could indicate that the plant is using the carbon dioxide produced by the snail for photosynthesis, balancing out the CO₂ levels.
- Test tube D (only water): This is the control test. Since there’s no plant or snail to produce or consume CO₂, any change in the indicator’s color would be due to external factors or the experiment’s set-up.
How will she know if she is correct?
Ananya will observe the color change of the carbon dioxide indicator in all the test tubes.
- Lets say if the water turns from yellow to blue, it indicates a decrease in carbon dioxide (likely due to photosynthesis).
- Lets say if the water turns from yellow to green or yellowish, it indicates an increase in carbon dioxide (likely due to respiration).
Q9: Design an experiment to observe if water transportation in plants is quicker in warm or cold conditions.
Ans: Aim : To observe if the rate of water transportation (transpiration and absorption) in plants is quicker in warm conditions compared to cold conditions.
Materials Needed:
- Two identical potted plants (same species and size)
- A source of water
- Two thermometers (for measuring temperature)
- A fan (for warm conditions, if necessary)
- A refrigerator (for cold conditions)
- Two transparent plastic bags (to cover the plants)
- Stopwatch or timer
Experimental Set-up:
Plant 1 (Warm Condition):
- Place one potted plant under a warm light source (like a lamp) or use a fan to maintain a warm temperature around the plant (around 28–30°C).
- Cover the plant with a transparent plastic bag to observe water droplets as the plant transpires.
- Place a thermometer near the plant to monitor the temperature.
Plant 2 (Cold Condition):
- Place the second potted plant in a cold environment, such as a refrigerator or a cooler with a temperature of around 10°C.
- Cover this plant with a transparent plastic bag as well to observe water droplets.
- Place a thermometer near this plant to monitor the temperature.
Procedure:
For both plants:
- Water both plants equally before starting the experiment.
- Place each plant in the respective environment (warm or cold).
- Monitor the temperature near the plants to ensure the conditions are maintained for at least 2–3 hours.
- Observe and record the amount of water condensation (water droplets) inside the plastic bag around the plant, which indicates transpiration.
- You may also choose to measure the water level in the soil before and after the experiment to observe the rate of water absorption.
- Observe for a set period, such as 2–3 hours, noting any changes in the water droplets in the plastic bag.
- Measure and compare the number of water droplets inside the plastic bag to see which plant shows more transpiration.
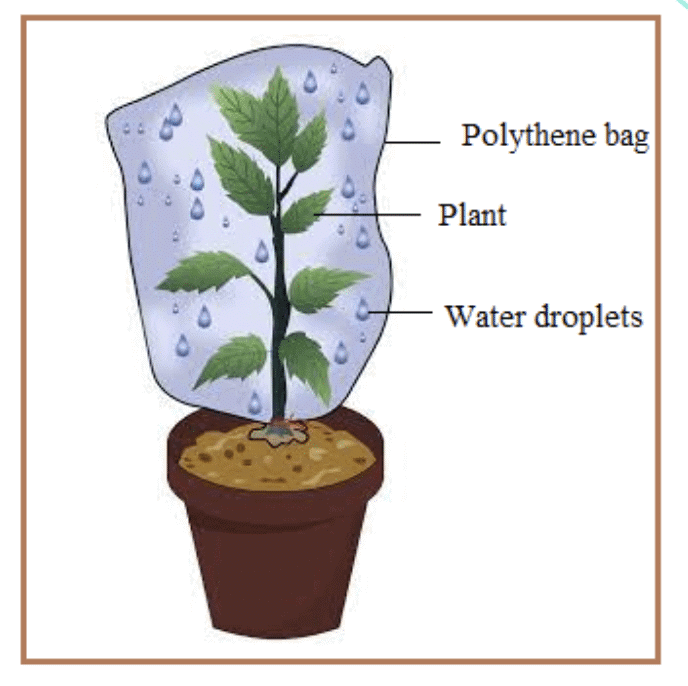
Observations:
- Plant 1 (Warm Condition): You will likely observe a higher number of water droplets inside the plastic bag in the warm environment, indicating a higher rate of transpiration.
- Plant 2 (Cold Condition): You will likely observe fewer water droplets or slower water loss in the colder environment.
Conclusion: The experiment will likely show that the plant in the warm condition will have a quicker rate of water transportation (transpiration and absorption) compared to the plant in the cold condition. This is because warmth causes the stomata to open more, increasing transpiration.
Q10:
Ans: Photosynthesis and respiration are crucial for maintaining the balance of gases in nature.
- Photosynthesis is the process by which plants convert sunlight, carbon dioxide, and water into glucose and oxygen. This process helps reduce carbon dioxide in the atmosphere and provides oxygen, which is essential for life.
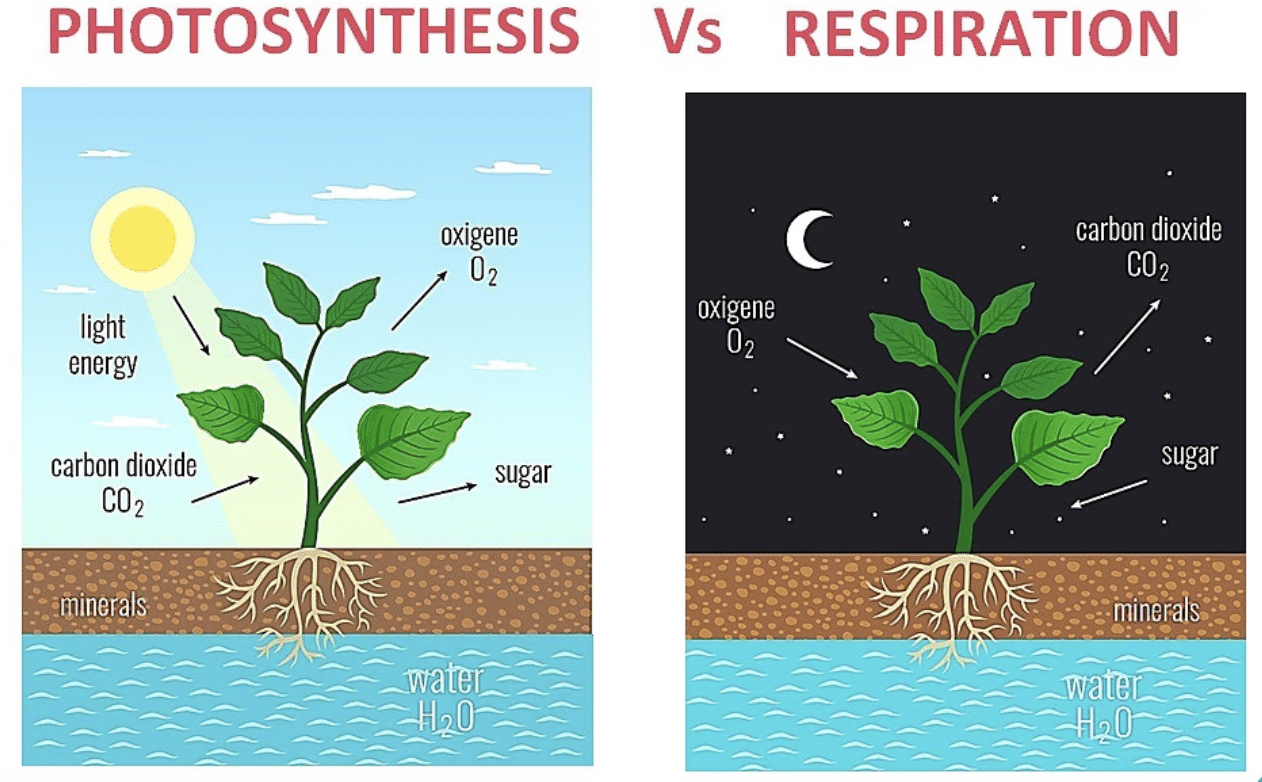 Respiration is the process where plants and animals break down glucose to release energy, consuming oxygen and releasing carbon dioxide. This helps maintain oxygen levels and provides carbon dioxide for plants to use in photosynthesis.
Respiration is the process where plants and animals break down glucose to release energy, consuming oxygen and releasing carbon dioxide. This helps maintain oxygen levels and provides carbon dioxide for plants to use in photosynthesis.
Together, these processes ensure a balance of oxygen and carbon dioxide in the atmosphere, which is vital for sustaining life on Earth.



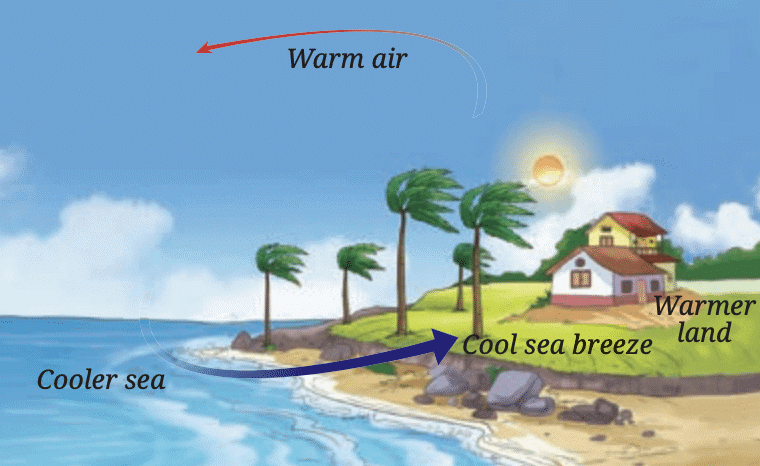 This cool air from the sea helps lower the temperature of the land near the water, preventing it from getting too hot.
This cool air from the sea helps lower the temperature of the land near the water, preventing it from getting too hot.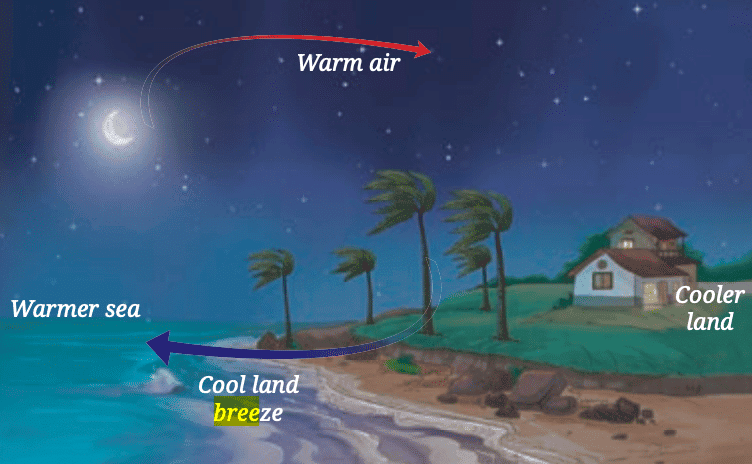 Thus at night, the water retains heat longer and warms the surrounding air, preventing it from getting too cold.
Thus at night, the water retains heat longer and warms the surrounding air, preventing it from getting too cold.
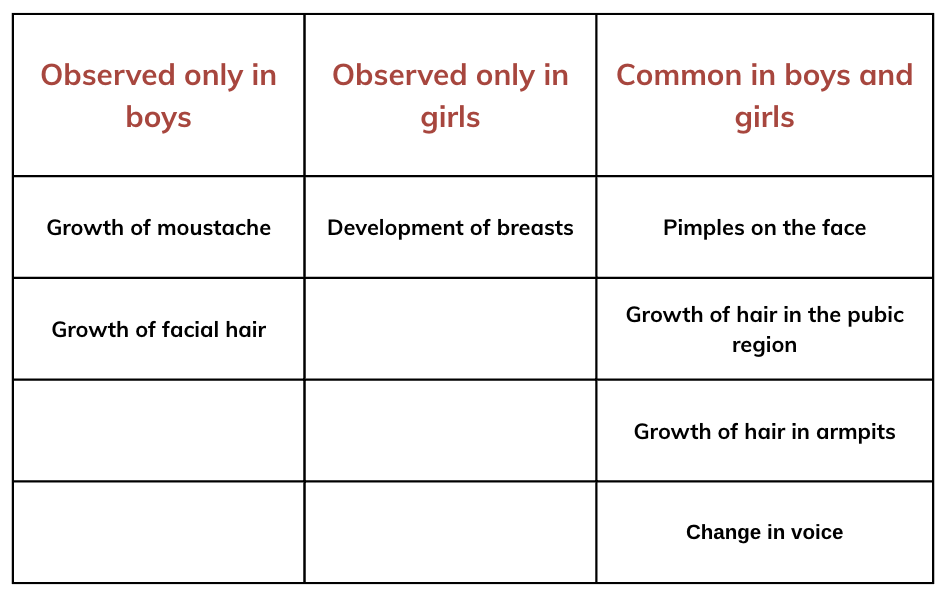
 Can you think of any examples where behavioral changes might affect the life of an adolescent?
Can you think of any examples where behavioral changes might affect the life of an adolescent? Changing Interests and Hobbies: Teenagers may suddenly develop new interests or abandon old ones, reflecting their changing personality and social environment.
Changing Interests and Hobbies: Teenagers may suddenly develop new interests or abandon old ones, reflecting their changing personality and social environment. For girls, maintaining hygiene during menstruation helps prevent discomfort, infections, and other health issues.
For girls, maintaining hygiene during menstruation helps prevent discomfort, infections, and other health issues.





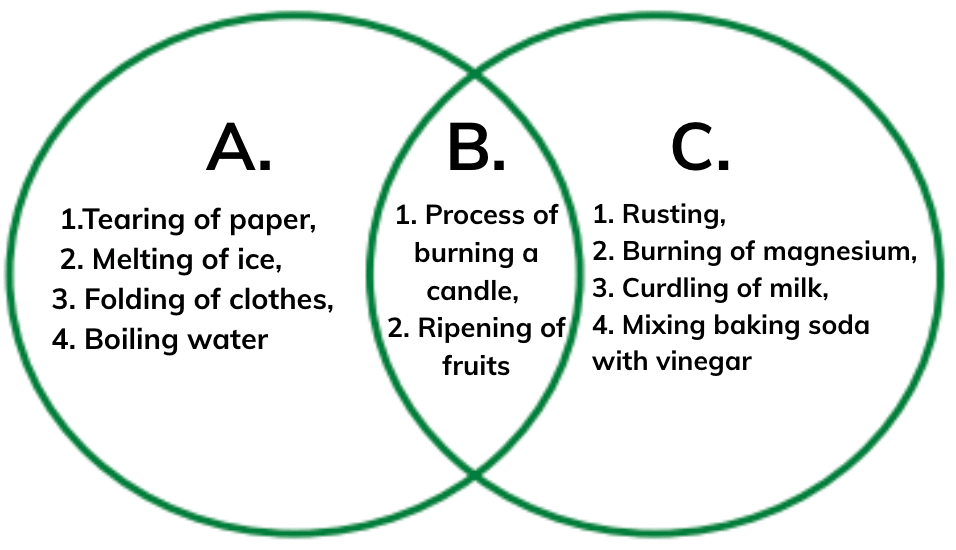


 Resistant to corrosion: They do not rust or tarnish easily, so the jewelry remains durable and keeps its shine over time.
Resistant to corrosion: They do not rust or tarnish easily, so the jewelry remains durable and keeps its shine over time.
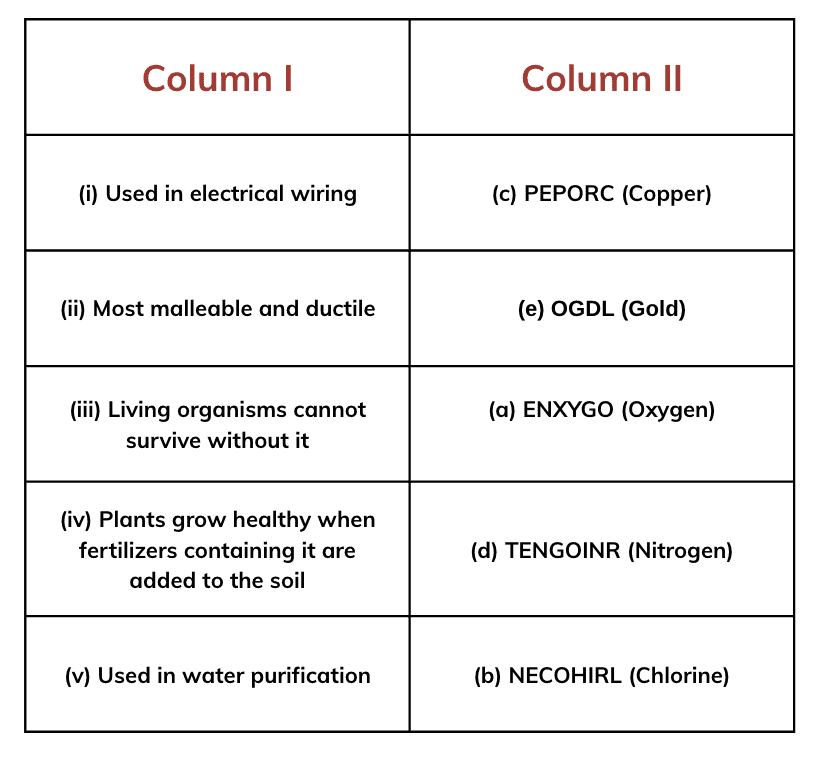

 Metals like copper and aluminum are excellent conductors of heat and electricity. This makes them ideal for use in wires, cooking utensils, and heating systems.
Metals like copper and aluminum are excellent conductors of heat and electricity. This makes them ideal for use in wires, cooking utensils, and heating systems.
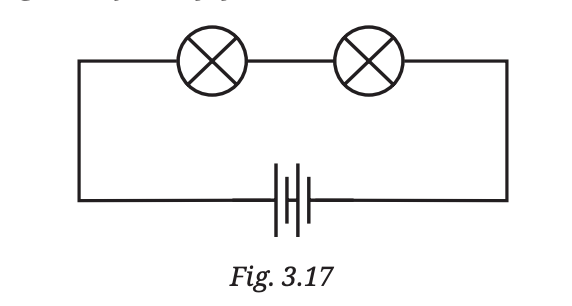
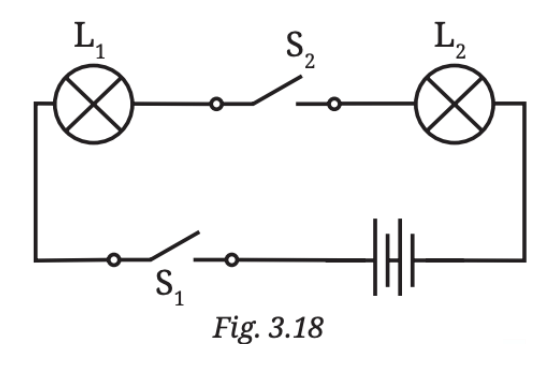
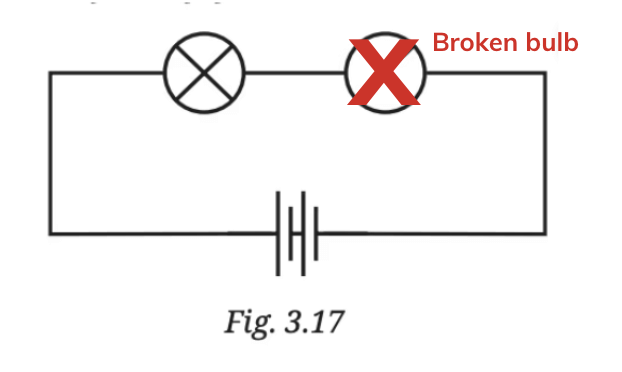 Even though the other lamp might be perfectly fine, it won’t glow because no electricity is reaching it due to the broken connection.
Even though the other lamp might be perfectly fine, it won’t glow because no electricity is reaching it due to the broken connection.
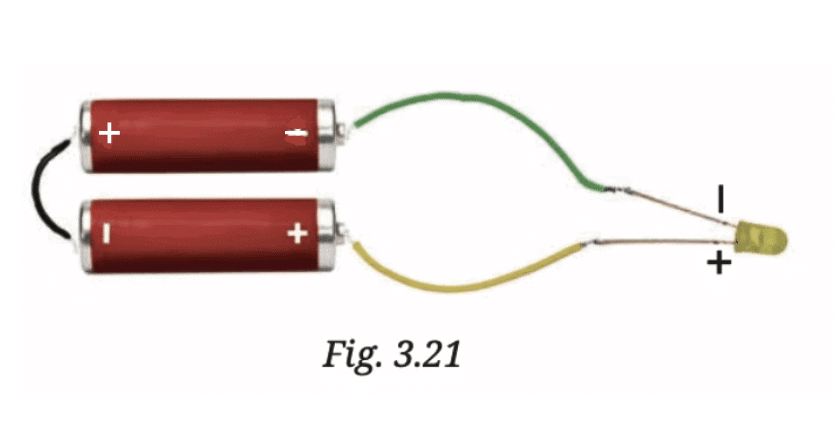
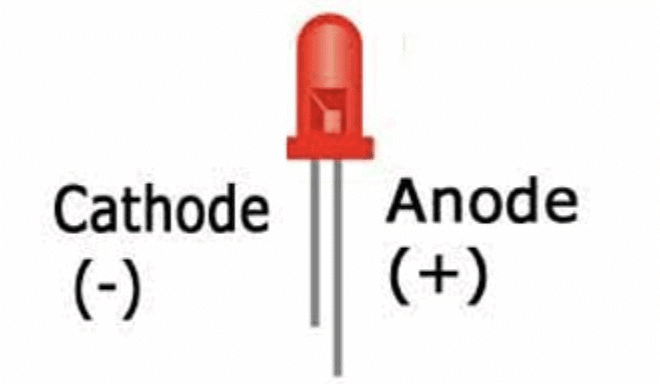 Shorter lead: This is the negative terminal (cathode).
Shorter lead: This is the negative terminal (cathode).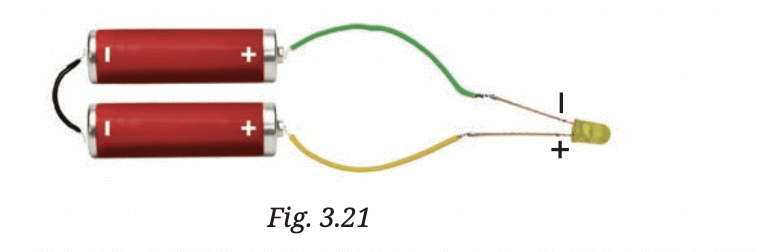

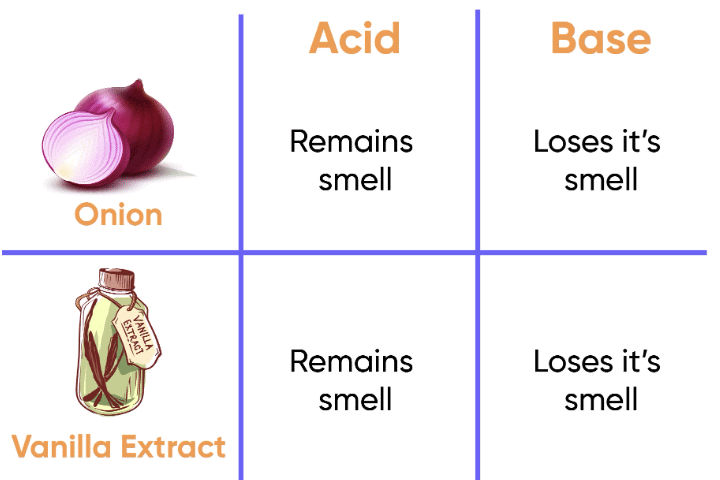 Onion and garlic have a strong smell that can sometimes change in the presence of bases, though they are not as commonly used as olfactory indicators.
Onion and garlic have a strong smell that can sometimes change in the presence of bases, though they are not as commonly used as olfactory indicators.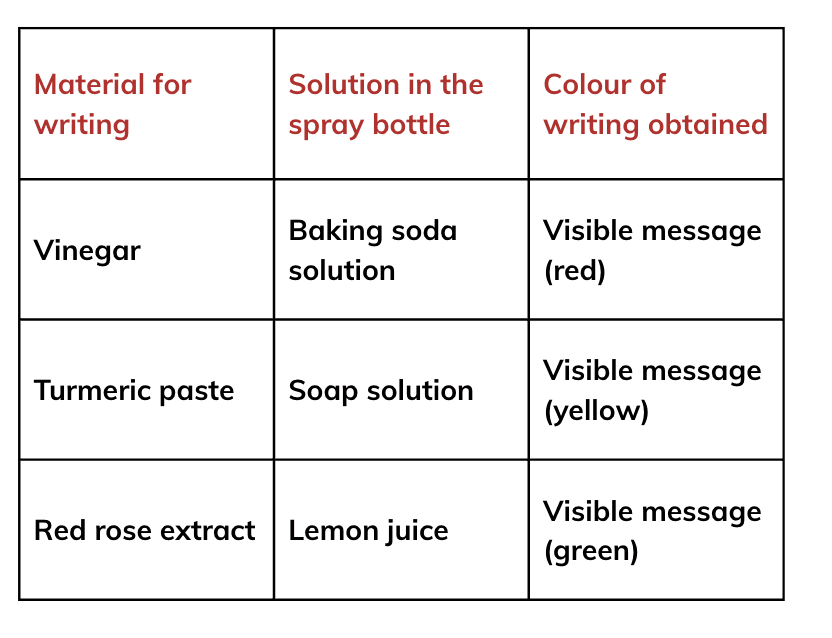
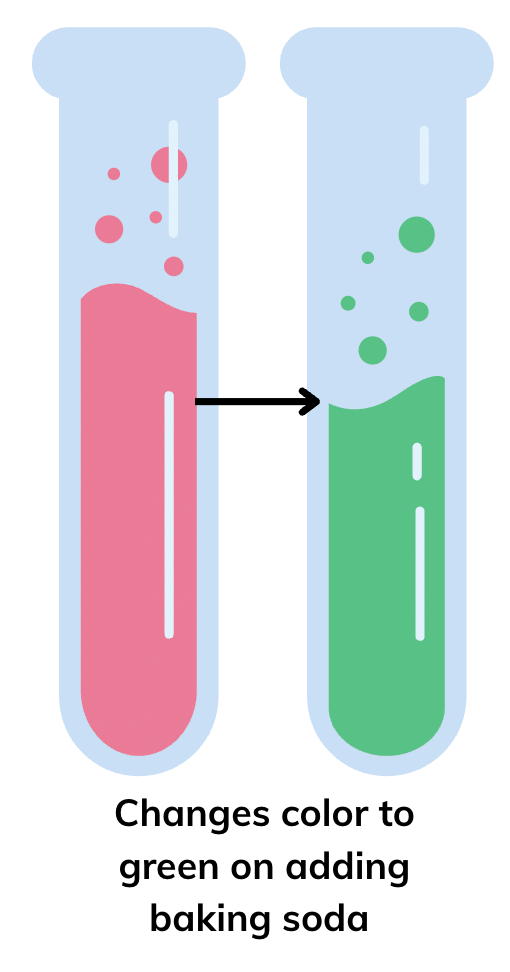


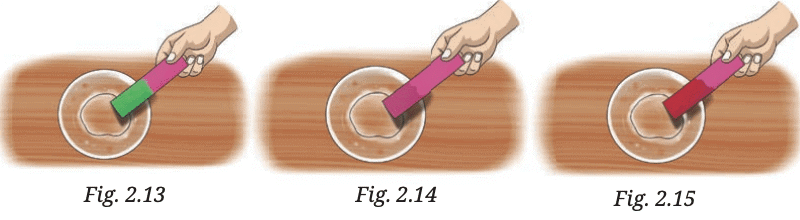
 So, if the turmeric paper stays yellow, the liquid is vinegar.
So, if the turmeric paper stays yellow, the liquid is vinegar. Since baking soda solution is basic, the turmeric paper will turn red, indicating that the liquid is a baking soda solution.
Since baking soda solution is basic, the turmeric paper will turn red, indicating that the liquid is a baking soda solution. If the turmeric paper remains yellow, the liquid is likely a sugar solution.
If the turmeric paper remains yellow, the liquid is likely a sugar solution.

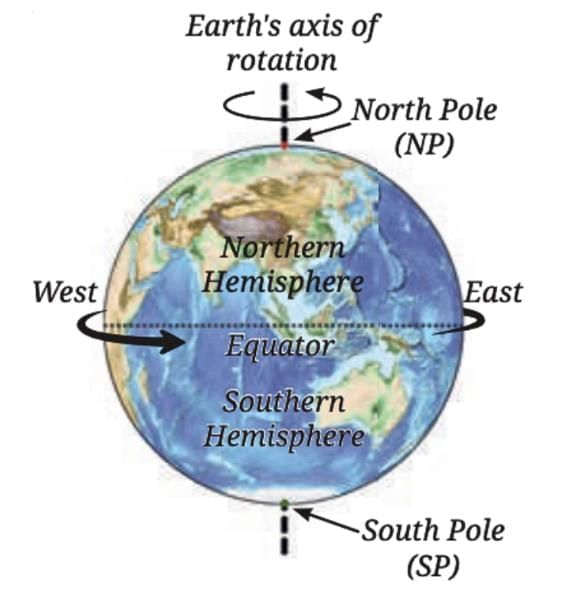
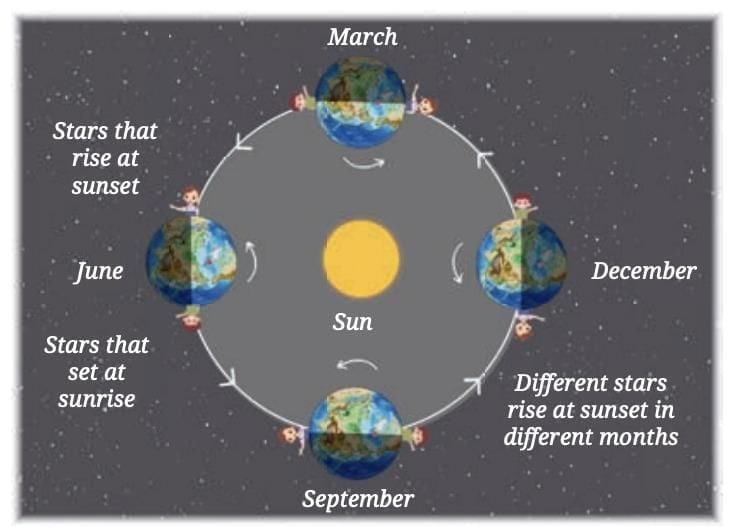 This causes the portion of the night sky visible after sunset to change gradually.
This causes the portion of the night sky visible after sunset to change gradually.
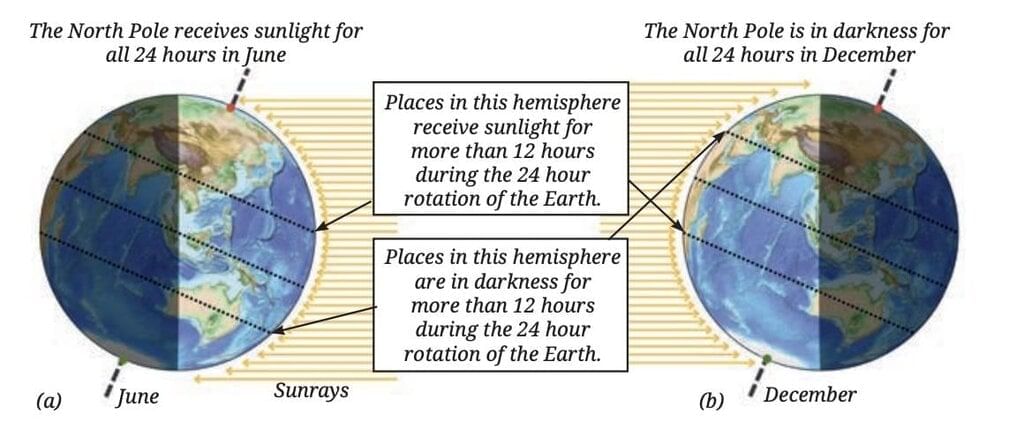
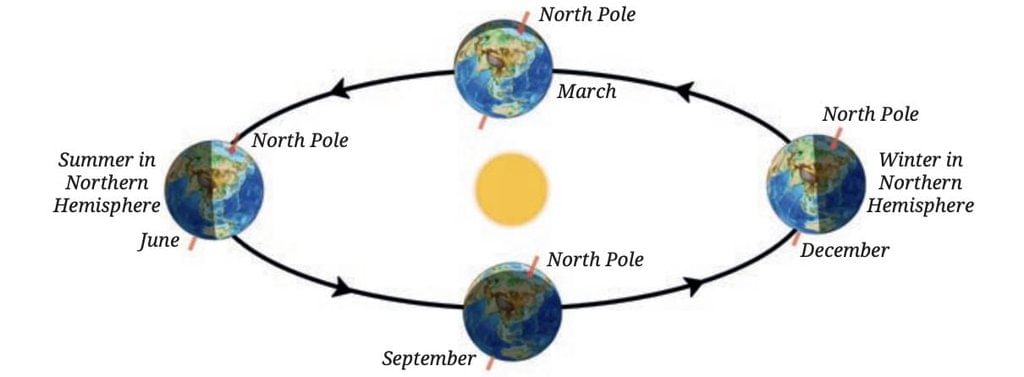 At the same time, the Southern Hemisphere tilts away from the Sun, getting less direct sunlight and shorter daylight hours, causing winter there.
At the same time, the Southern Hemisphere tilts away from the Sun, getting less direct sunlight and shorter daylight hours, causing winter there.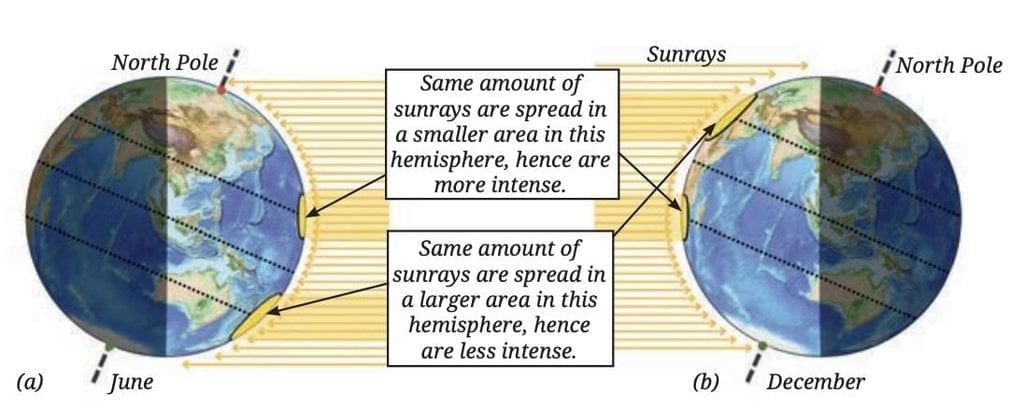
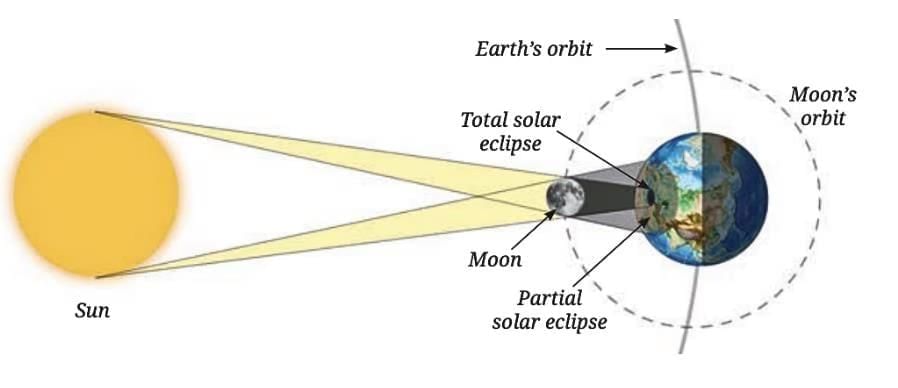



 Projecting the Sun’s image using a mirror to watch the eclipse safely.
Projecting the Sun’s image using a mirror to watch the eclipse safely.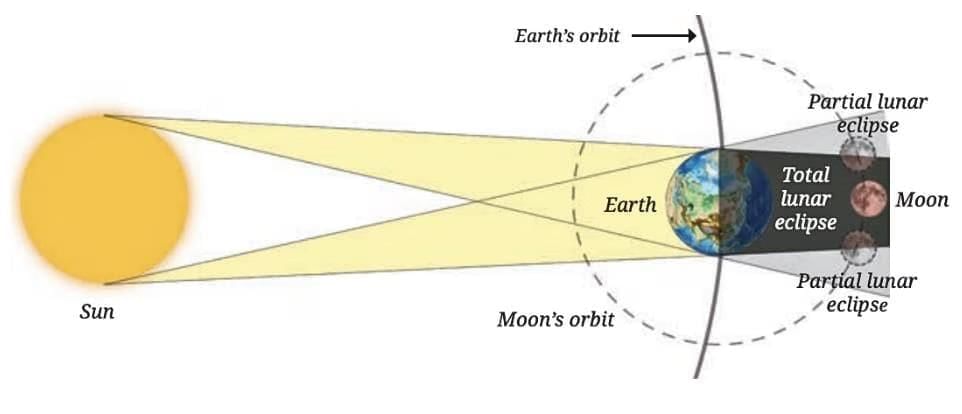

 M.K. Vainu Bappu is regarded as the father of modern Indian astronomy. He led efforts to set up many astronomical instruments and telescopes in India, such as those at Manora Peak (Uttarakhand) and Kavalur (Tamil Nadu).
M.K. Vainu Bappu is regarded as the father of modern Indian astronomy. He led efforts to set up many astronomical instruments and telescopes in India, such as those at Manora Peak (Uttarakhand) and Kavalur (Tamil Nadu).