Chapter Notes: Our Home: Earth, a Unique Life-Sustaining Planet
What would Earth be like if it had never known life at all?
Our planet is not just another ball of rock in space. It is the only known place in the universe that supports a vast variety of life — from towering mountains to deep oceans and green forests. With the help of Earth observation satellites such as those operated by ISRO, scientists study the special features that make Earth a life‑sustaining planet.
 Image by Earth Observation Satellite (ISRO)
Image by Earth Observation Satellite (ISRO)
In this chapter you will explore the conditions and systems that make Earth uniquely fit for life. You will review Earth’s physical structure, its place in the Solar System, how living and non‑living things interact, and the challenges that threaten life on Earth.
About the Image:
- The image was captured by an ISRO Earth Observation Satellite.
- It is a mosaic made by combining nearly 3,000 smaller images.
- This is a false‑colour image — special colours are used to highlight specific information.
- Such images help scientists study land, water, plant growth, and environmental changes more clearly.
Why Is Earth a Unique Planet?
Out of the billions of planets in the universe, Earth is the only one we know that has life in so many forms — plants, animals, people, and microorganisms.
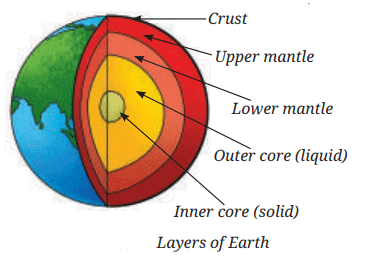
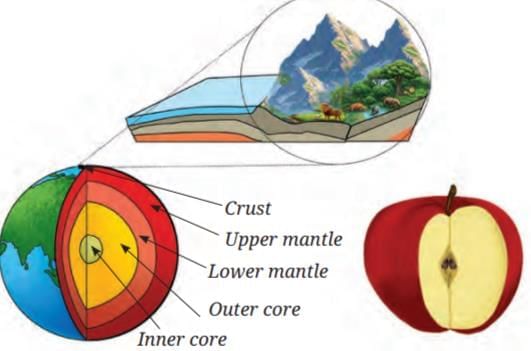 Earth’s Crust is like the thin skin of an apple
Earth’s Crust is like the thin skin of an apple
- All living things exist on a very thin layer on Earth’s surface called the crust.
- If Earth were the size of an apple, the crust where life exists would be as thin as the apple’s skin.
- Below the crust there are other layers: upper mantle, lower mantle, outer core, and inner core, but life is restricted mostly to the crust and the near‑surface parts of the mantle and atmosphere.

Activity 13.1: What Makes Earth Special?
Think about and list interesting features of Earth that matter for our lives. For example:

Here is a completed table you can discuss and add to:
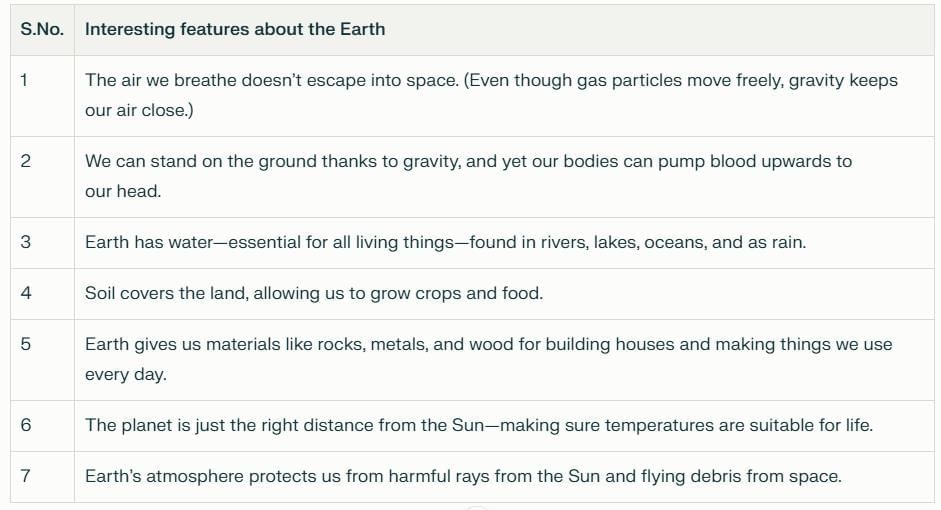
- Although the crust is thin, it provides everything needed for life: air, water, soil, and resources.
- Earth supplies air to breathe, water for drinking and agriculture, and soil for growing food.
- Materials such as rocks, timber, and metals are available for building homes, roads and tools.
Planets of Our Solar System
The Solar System has eight planets that orbit the Sun in nearly circular paths.
Planets in order from the Sun:
- Mercury
- Venus
- Earth
- Mars
- Jupiter
- Saturn
- Uranus
- Neptune
Types of planets:
- Mercury, Venus, Earth, Mars: Smaller, rocky (terrestrial) planets.
- Jupiter, Saturn, Uranus, Neptune: Larger, mostly made of gases and ices (gas giants and ice giants).
Activity 13.2: Comparing Planets
Collect and compare information about:
- The average temperature of each planet.
- Relative size (radius) compared to Earth.
- Whether it has an atmosphere.
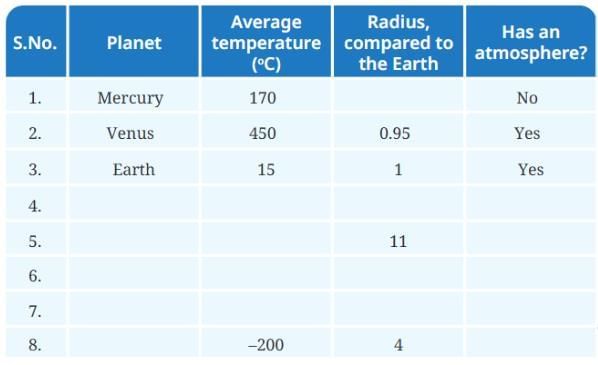
Observations:
- All planets receive energy from the Sun.
- Planets closer to the Sun are generally hotter; those further away are colder.
Why Is Venus the Hottest Planet?
- Venus is not the closest planet to the Sun, but it is the hottest.
- Its atmosphere is very thick and is made mainly of carbon dioxide (CO2).
- Carbon dioxide traps heat through the greenhouse effect, so Venus retains large amounts of heat.
- On Earth, the greenhouse effect also warms the surface, but it is much weaker and essential for maintaining temperatures suitable for life.
Difference between planetary greenhouse and a plant greenhouse:
- Planetary greenhouse: Gases in the atmosphere trap infrared radiation (heat) emitted from the surface.
- Plant greenhouse: Glass walls reduce air circulation and trap warm air physically.
- Both keep places warm, but the mechanisms differ.
What Makes the Earth Suitable for Life to Exist?
Several physical and chemical properties of Earth combine to make it habitable. These include its position in the Solar System, size and atmosphere, and its magnetic field.
1. Position of Earth — The
Habitable Zone
Earth’s distance from the Sun is just right — neither too close nor too far.
- This distance keeps temperatures such that water mostly exists as a liquid, which is essential for all known life.
- If Earth were closer to the Sun, it would be too hot and water would evaporate.
- If Earth were further from the Sun, it would be too cold and water would freeze.
- The region around a star where liquid water can exist is called the habitable zone or the Goldilocks zone.
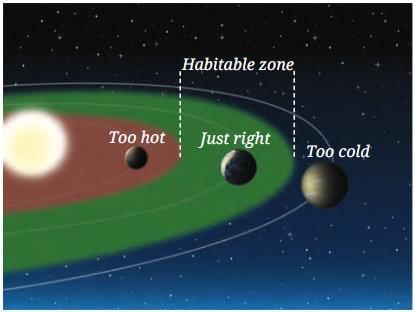 Habitable Zone
Habitable Zone - Over 70% of Earth’s surface is covered with water, giving Earth the name the Blue Planet.

Blue Planet
Mars and the Possibility of Life
 Mars
Mars
- Mars lies near the edge of the Sun’s habitable zone.
- Many spacecraft and rovers have studied Mars; no proof of current life has been found yet.
- Evidence suggests Mars may once have had liquid water and possibly simple life. Future missions may provide new information.
2. Size of Earth and Its Atmosphere
Earth’s nearly circular orbit helps keep sunlight and temperatures fairly steady throughout the year.
- If Earth were smaller, its gravity might be too weak to hold a dense atmosphere; gases could escape to space (as on Mercury and Mars).
- If Earth were much larger, very strong gravity might make conditions unsuitable for life as we know it.
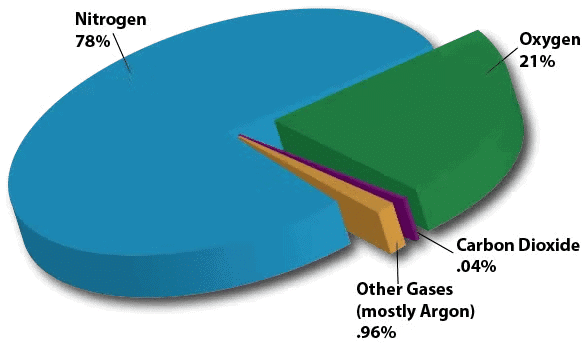
- The atmosphere supplies oxygen for breathing and carbon dioxide for plants.
- Some oxygen in the upper atmosphere forms ozone, creating the ozone layer that shields life from harmful ultraviolet (UV) radiation.
- The atmosphere also contributes to the greenhouse effect, which traps enough heat to keep Earth warm but not too hot.
Our Scientific Heritage: Exploring Mars
 Mangalyaan
Mangalyaan
- Mangalyaan (Mars Orbiter Mission), launched by ISRO in 2013, is an important Indian mission to study Mars.
- The mission studied Mars’ atmosphere and surface and searched for signs of past water and conditions that could have supported life.
- Mangalyaan demonstrated how effective, low‑cost space missions can deliver valuable scientific results.
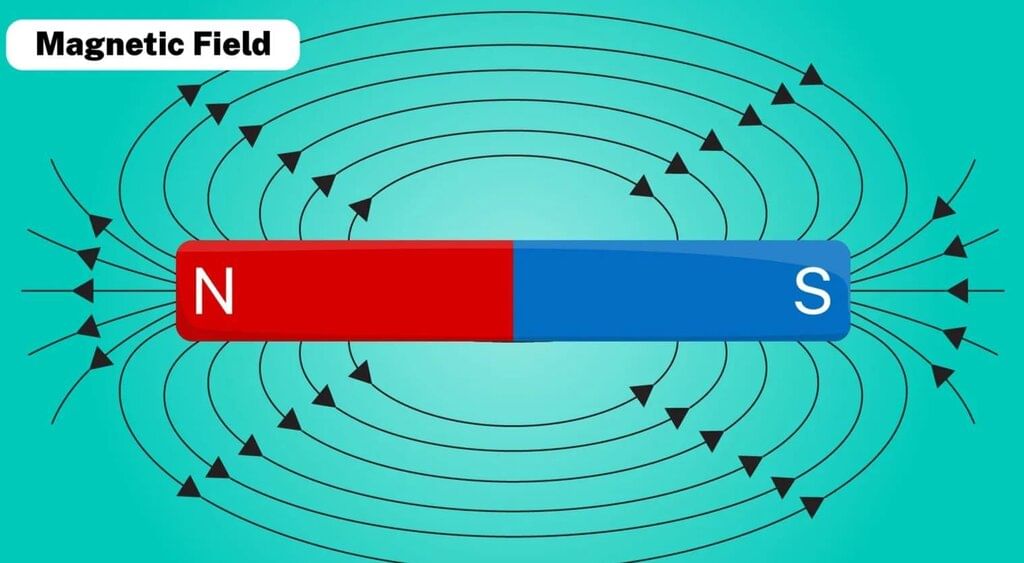
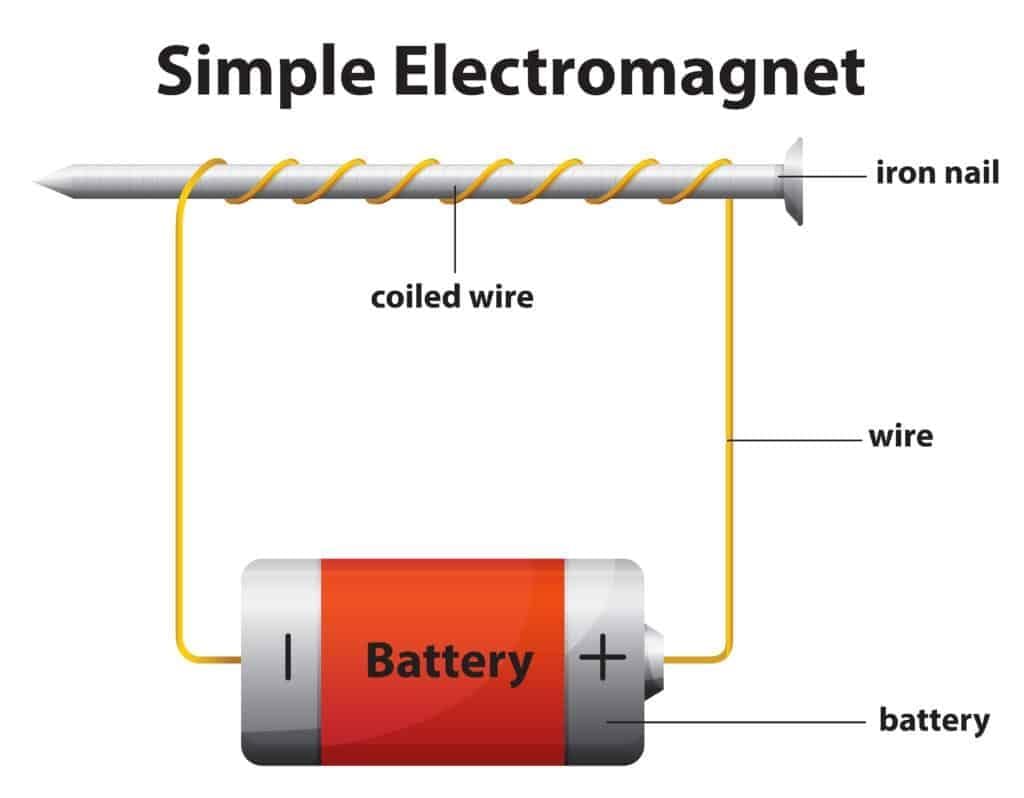
3. Magnetic Field of the Earth
Along with Earth’s right position, size, and atmosphere, the magnetic field is another key factor that helps make Earth safe for life.
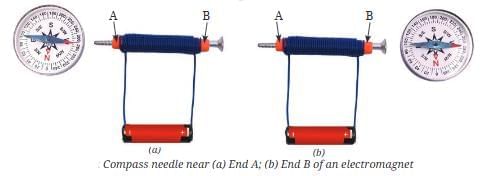
- Earth behaves like a giant magnet; a freely suspended magnet (a compass needle) points toward the magnetic north and south.
- The region around a magnet where its influence is felt is called the magnetic field.
- Scientists believe the motion of molten iron in the Earth’s outer core generates the magnetic field.
Why Earth’s Magnetic Field Is Important
Earth is constantly hit by tiny, high‑energy particles from space:
- Cosmic rays from distant space.
- Solar wind, composed of charged particles emitted by the Sun.
- These particles can damage the atmosphere, reduce the ozone layer, increase harmful UV radiation and harm living organisms.
- The magnetic field acts as a shield by deflecting many of these charged particles away from Earth.
- This protection helps preserve the atmosphere and makes Earth safer for life.

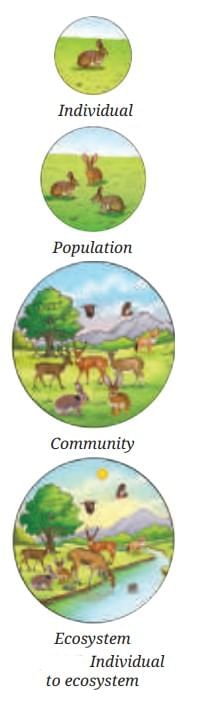
What Allows Life to Be Sustained on Earth?
It is the connections between living (biotic) and non‑living (abiotic) parts of Earth that enable life to thrive.
Air, Water and Sunlight
- The atmosphere provides oxygen for respiration and contains carbon dioxide used by plants for photosynthesis.
- Sunlight supplies energy for photosynthesis and warms Earth’s surface. Part of this heat is trapped by the atmosphere (greenhouse effect), keeping temperatures suitable for liquid water.
- Water covers nearly 70% of Earth and forms the hydrosphere(oceans, seas, rivers, lakes, groundwater). Water:
- dissolves and transports nutrients,
- helps animals regulate body temperature and digestion,
- is essential for photosynthesis and all cellular processes.
- Freshwater is required for agriculture and human consumption.
- Water vapour forms clouds that produce rain and snow, which refill rivers, lakes, and groundwater.
- Air movement (wind) shapes weather and rainfall patterns; this influences farming and water supply.
Soil, Rocks and Minerals
- The geosphere (Earth’s crust) contains rocks, soil and minerals that support life.
- Soil provides a medium for plant growth and contains nutrients such as nitrogen and potassium, released by weathering of rocks and decomposition of dead organisms.
- Minerals in rocks and soil are resources for salt, coal, oil, iron, copper and many other materials essential for human life and technology.
- Geodiversity — a variety of landforms, rocks and soils — creates different habitats and supports biodiversity.
- Non‑living parts of nature actively shape ecosystems and biological communities.
Plants, Animals and Microorganisms
The biosphere is the zone where life exists — including land, water and the lower atmosphere. It contains all living organisms: trees, grasses, herbs, animals, insects and microscopic organisms such as bacteria and fungi.
- Plants perform photosynthesis, using sunlight, water and carbon dioxide to make food and release oxygen.
- Animals depend on plants and other animals for food and energy.
- Microorganisms (decomposers) break down dead matter and recycle nutrients into the soil for use by plants.
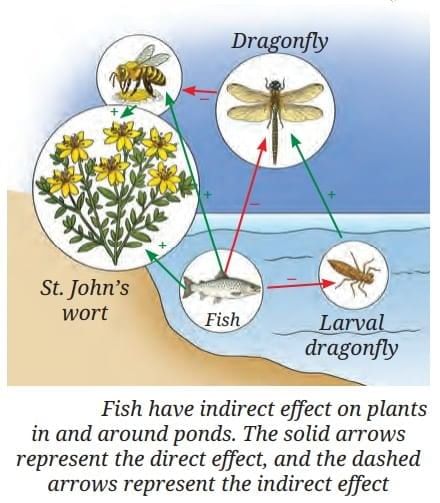
- These interactions form complex food chains and food webs that maintain ecological balance — the essence of ecology.
The Importance of Balance
- Earth is an interconnected system: land, air, water and living organisms interact constantly.
- Small changes — such as deforestation — can alter rainfall, soil quality, air composition and wildlife populations.
- Maintaining balance in these systems is essential for a healthy, habitable planet.
- Protecting clean air, water, soil and biodiversity helps secure Earth’s future.
What Keeps Life from Disappearing?
If organisms did not reproduce, species would eventually disappear. Reproduction ensures continuity of life across generations.
What is Reproduction?
- Reproduction is the biological process by which organisms produce new individuals of the same kind.
- Parents pass down instructions called genes (genetic material) that determine the form and functioning of an organism.
- Genes are found in cells and guide the development of body parts and biological processes.
Why is Reproduction Important?
- It keeps each species going generation after generation.
- It allows variation — small changes in genes — that can help organisms adapt to changing environments.
- Accumulation of such changes over many generations can lead to evolution of new traits or species.
- Examples: Camels developed humps to survive desert conditions; microbes can evolve antibiotic resistance.
How Can Offspring Be Similar Yet Different?
Reproduction can produce offspring that resemble their parents yet display differences.
There are two main types of reproduction:
- Asexual reproduction: New individuals arise from a single parent and are genetically almost identical to it.
- Sexual reproduction: Two parents contribute genetic material, producing offspring with mixed traits from both parents.
Asexual Reproduction
In asexual reproduction, one parent produces new individuals that are genetic copies of the parent.
Example: Many plants can reproduce by vegetative propagation — planting a part of the plant (leaf, stem, root) and growing a new plant.
Activity 13.3: Vegetative Propagation in Plants
- Plant stem cuttings (e.g. money plant), potato eyes, or pieces of ginger in moist soil.
- Provide water, air and sunlight.
- Observe daily when roots, shoots and new leaves appear.
- This demonstrates how some plants grow new individuals from a part of the original plant.
- Other examples of asexual reproduction:
- Bacteria, amoeba: Binary fission — one cell divides into two identical cells.
- Hydra: Budding — a new individual grows from the body and detaches.
- Planaria, some algae: Regeneration — they can regrow from fragments.
Sexual Reproduction
Sexual reproduction is the process in which two parents (usually male and female) produce offspring.
This is common in most animals and flowering plants. Some microorganisms also have mating types that act like parents.
Special Cells for Reproduction: Gametes
Both parents produce special reproductive cells called gametes.
- Male gametes: sperm in animals; pollen in flowering plants.
- Female gametes: egg in animals; ovule in plants.
- Gametes carry half the genetic information of each parent. When a male and female gamete fuse (fertilisation), they form a zygote with a full set of genes.
Why Don’t Babies Look Exactly Like Their Parents?
- Offspring receive a unique mix of genes from both parents.
- That is why they resemble their parents but are not exact copies.
- Siblings may look different because each receives a different combination of genes.
- Mixing of genes increases variation, which is useful for adaptation and evolution.
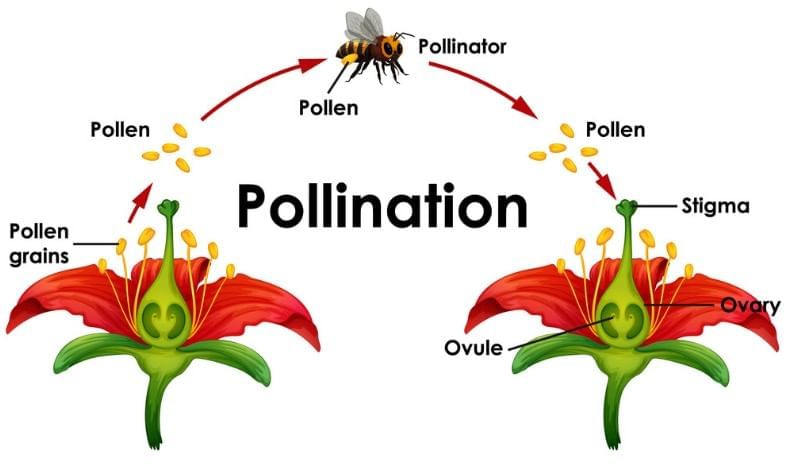
Sexual Reproduction in Plants
Flowering plants have male and female parts:
- Anther (male) produces pollen grains (male gametes).
- Ovule (female), inside the ovary, contains the female gamete.
- Pollination: Transfer of pollen from anther to stigma, often by wind, insects or animals.
Fertilisation: Pollen reaches the ovule and male and female gametes fuse to form a zygote.
- The zygote develops into a seed.
- The ovule becomes the seed and the ovary develops into fruit.
Seed dispersal: Fruits or seeds are carried away by animals, wind or water. Seeds that fall in suitable places germinate using stored food to grow roots and shoots.
Sexual Reproduction in Animals
Animals have two types of reproductive cells: sperm (male) and egg (female).
- Fertilisation occurs when sperm and egg unite to form a zygote.
- In fish and amphibians (e.g. frogs), fertilisation often takes place externally in water, with parents releasing eggs and sperm into water.
- In birds and most mammals, fertilisation occurs inside the female’s body. Sperm swim to meet the egg.
After fertilisation:
- Birds lay eggs; the embryo develops using food stored in the egg until hatching.
- Mammals (most) give birth to live young; the embryo develops inside the mother who supplies food and oxygen.
- Main difference:
- Egg‑laying animals provide food for the embryo inside the egg.
- Mammals provide food to the embryo inside the mother’s body.
What Are the Threats to Life on Earth?
Earth’s life depends on a delicate balance between living things (plants, animals, microbes) and non‑living things (air, water, soil, sunlight). Human activities are disturbing this balance, leading to major environmental problems.
The three main global challenges today are:
- Climate change
- Biodiversity loss
- Pollution
1. Climate Change
- Burning fossil fuels (coal, oil, gas) releases greenhouse gases like carbon dioxide (CO2) and methane.
- These gases trap more heat in the atmosphere, causing global warming.
- Normally, CO2 is absorbed by plants, trees and plankton in oceans, but excess CO2 from burning fossil fuels adds more heat than the Earth can absorb quickly.
- Even a small temperature rise can:
- melt ice caps and raise sea levels, causing coastal flooding,
- increase extreme weather events such as heavy rainfall, storms, droughts and heat waves,
- cause extinction of plants and animals that cannot adapt quickly.
- Long‑term changes in temperature, rainfall and weather patterns are collectively known as climate change.
2. Biodiversity Loss
- Destroying habitats (forests, grasslands, wetlands) causes plants and animals to vanish.
- This upsets food chains and ecosystems:
- If grasses disappear, herbivores lose food.
- Without herbivores, predators cannot survive.
- Each species plays a role; losing species weakens nature’s capacity to support life.
3. Pollution
Air pollution
- Comes from factories, vehicles and burning fuels.
- Harms human health (respiratory illness), damages crops and causes smog and acid rain.
Water and soil pollution
- Caused by industrial effluents, agricultural chemicals and plastic waste.
- Harms aquatic life, makes water unsafe, and reduces crop yields.
- Polluted soil can introduce toxins into the food chain.
All these problems affect people, animals, plants and ecosystems.
The Importance of Global and Local Action
- Small changes in global temperature, atmospheric composition or ozone can endanger life.
- Earth’s systems — hydrosphere (water), biosphere (living things), atmosphere (air) and geosphere (rocks and soil) — are connected; harm to one affects the others.
International Agreements and Efforts
Countries have agreed on treaties to protect Earth:
- Montreal Protocol (1987): Reduced chemicals (CFCs) that damaged the ozone layer; helped ozone recovery.
- Earth Summit (1992): A global meeting that promoted cooperation on environment and development.
- Kyoto Protocol (entered into force 2005): Set binding emission reduction targets for some countries.
- Paris Agreement (2015): Countries committed to limit global warming; the goal is to keep warming well below 1.5°C above pre‑industrial levels.
- As of 2025, the world has not yet achieved the 1.5°C goal — stronger action is still needed.
How Can We Help? — Individual and Local Actions
- Cut down on pollution: Avoid burning waste; reduce vehicle emissions.
- Switch to cleaner energy: Use solar, wind and other renewable sources instead of coal and oil.
- Use energy and water carefully: Turn off lights, repair leaks, and use public transport or cycle where possible.
- Reduce, reuse and recycle: Repair items instead of throwing away; recycle paper, plastic, glass and metal.
- Practice sustainable farming and waste management: Use organic methods, reduce chemical use and manage sewage properly.
- Protect biodiversity: Conserve habitats, plant native trees and support protected areas.
- Community action: Local communities managing natural resources wisely can make a large positive difference.
Final Summary
- Earth is uniquely suited for life because of its right distance from the Sun, suitable size and gravity, atmosphere and magnetic field.
- Life depends on continuous interactions among air, water, soil, rocks and living organisms.
- Reproduction — both asexual and sexual — ensures continuation and variation of life.
- Human activities have introduced serious threats: climate change, biodiversity loss and pollution.
- Global treaties and local actions together can help protect Earth; everyone can contribute through small, practical steps.
 Elephants moving in search of food and shelter
Elephants moving in search of food and shelter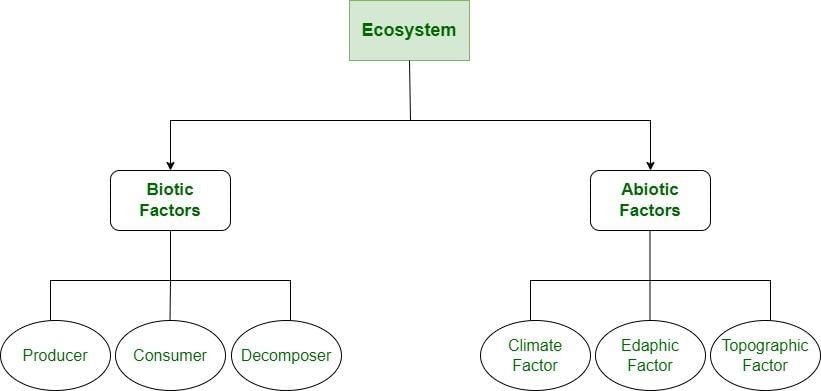

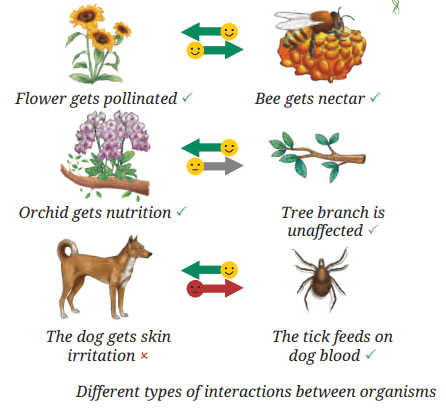
 What Does This Show?
What Does This Show?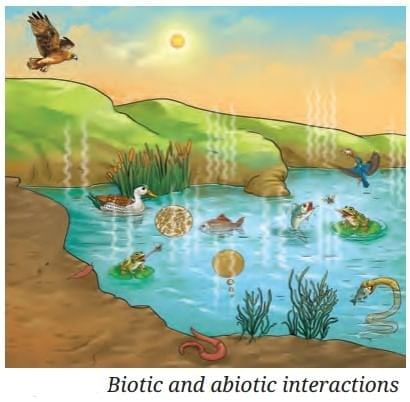

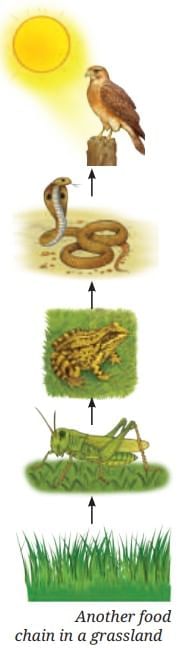 Trophic Levels
Trophic Levels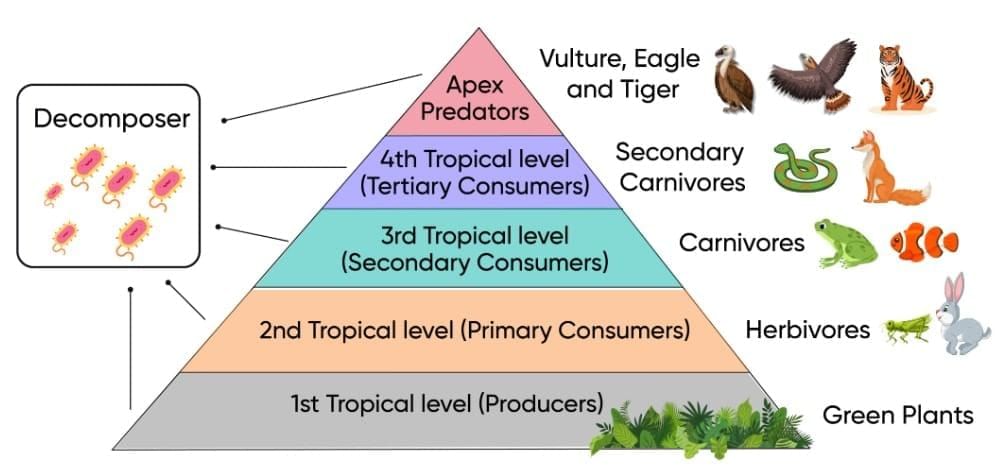 Trophic Level Pyramid
Trophic Level Pyramid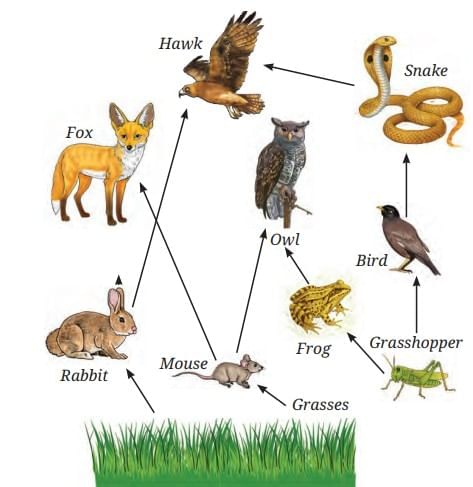 Food Web
Food Web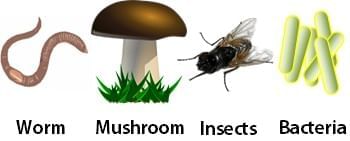 Examples of Decomposers
Examples of Decomposers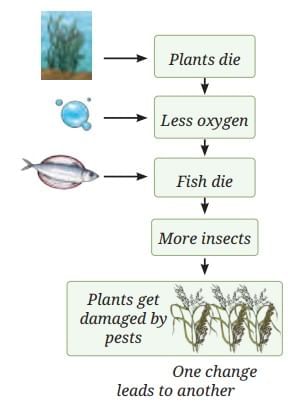



 Kites in the Sky
Kites in the Sky


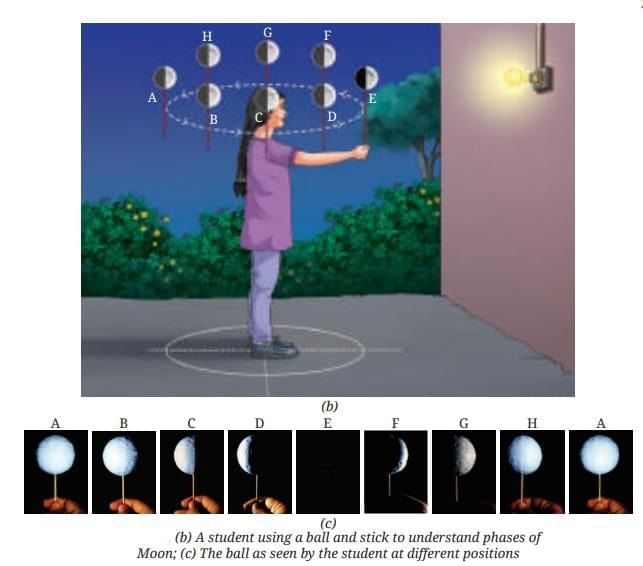
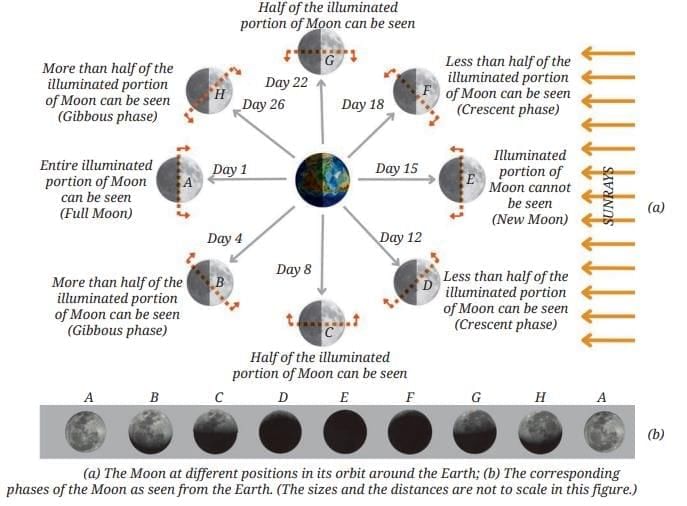
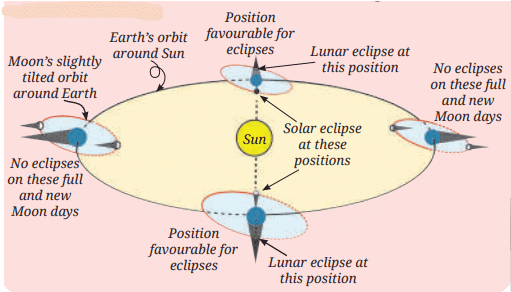
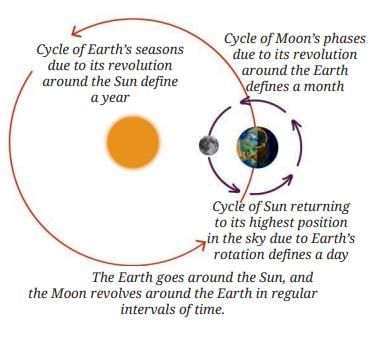


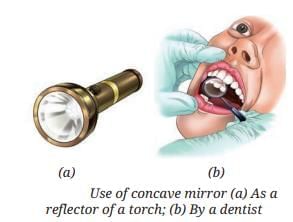
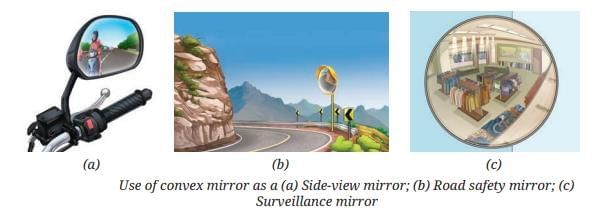
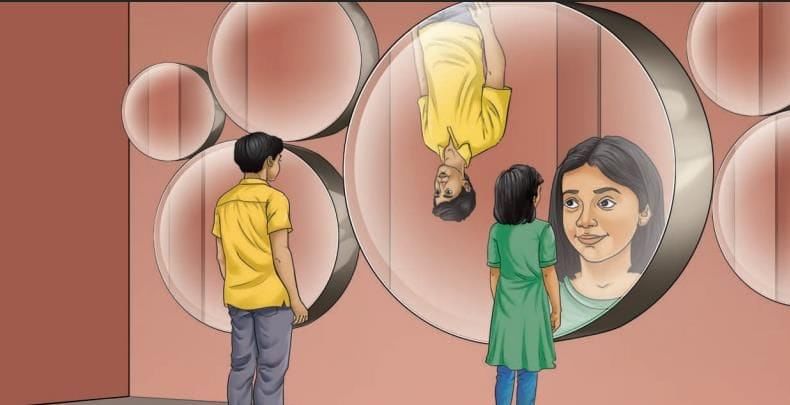 MirrorsTo her surprise, when she looked into one, her face seemed comically large, while her brother, just a little farther away, looked upside down! At another mirror, she saw a tiny version of herself staring back.
MirrorsTo her surprise, when she looked into one, her face seemed comically large, while her brother, just a little farther away, looked upside down! At another mirror, she saw a tiny version of herself staring back.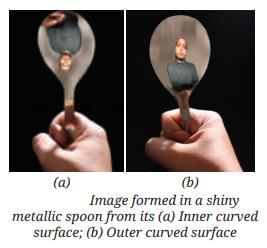
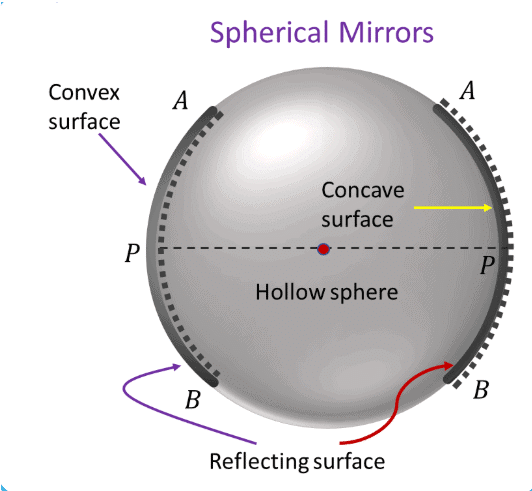
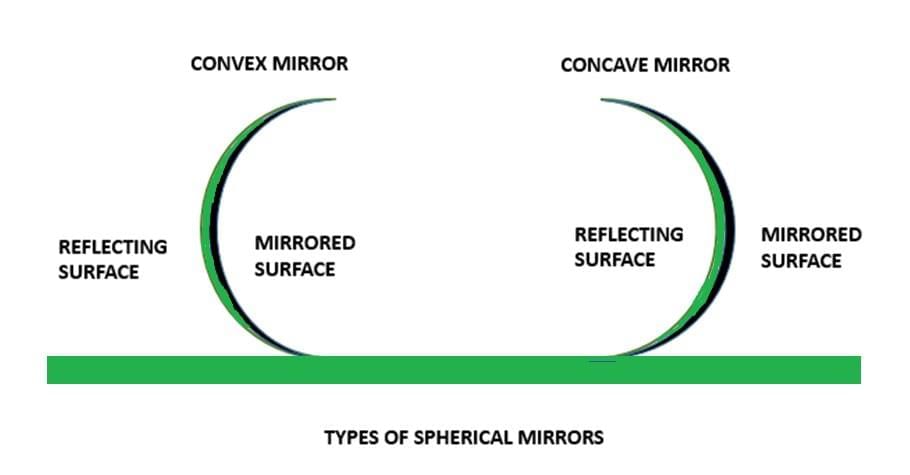
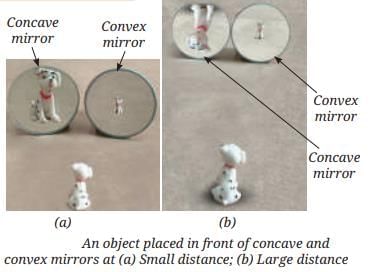
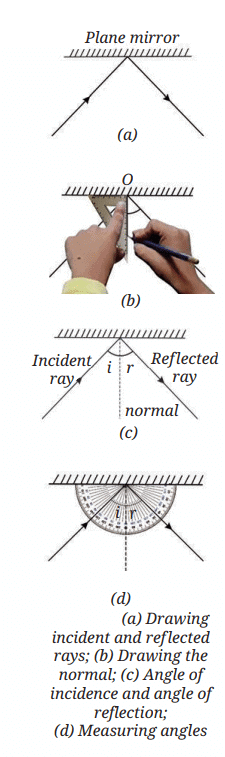
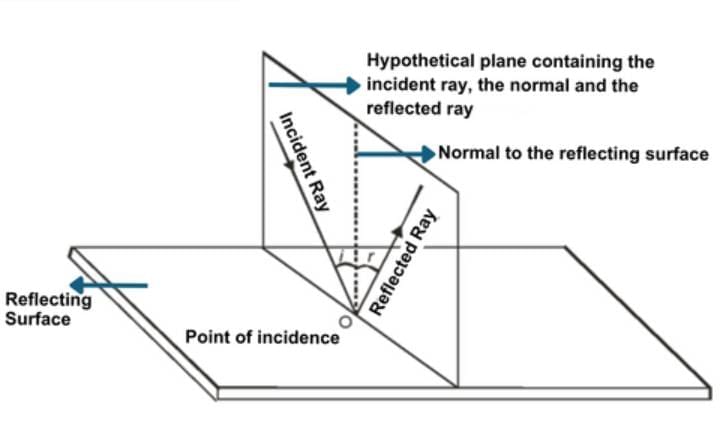
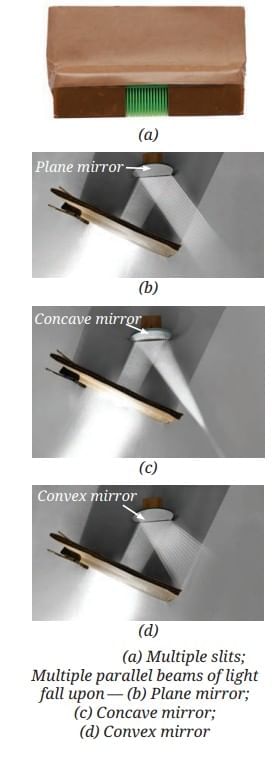
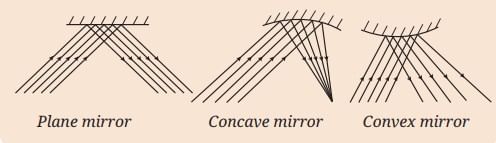
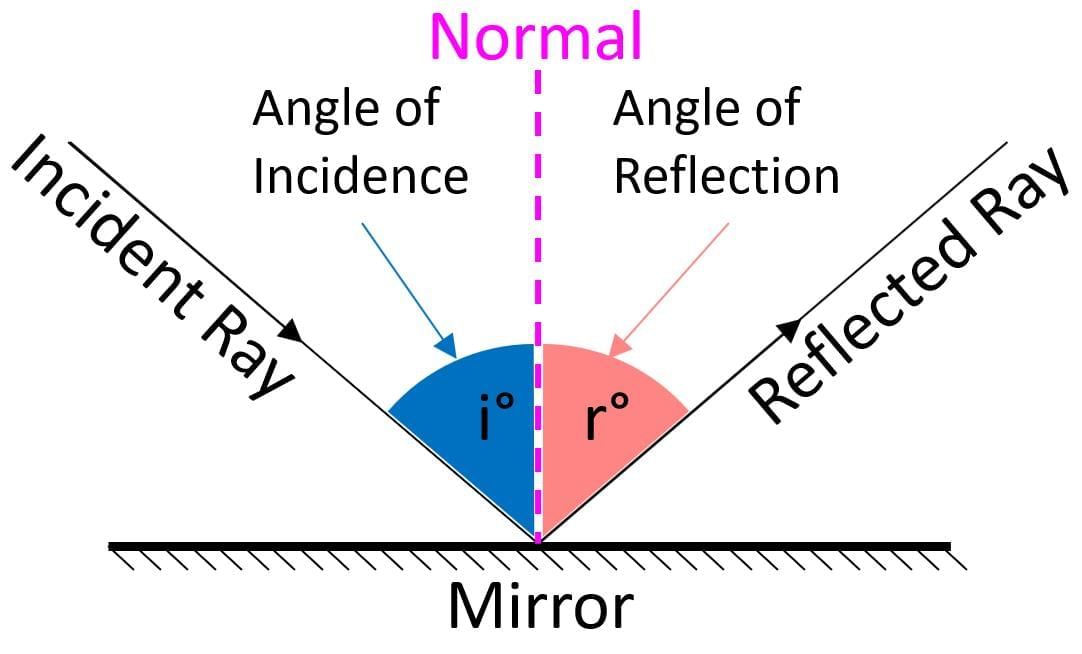 First Law of Reflection
First Law of Reflection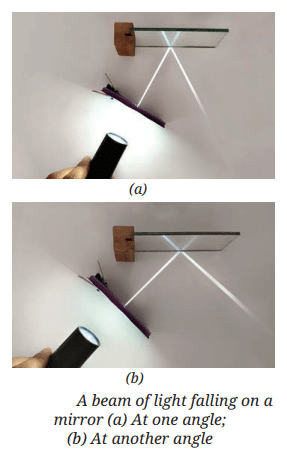



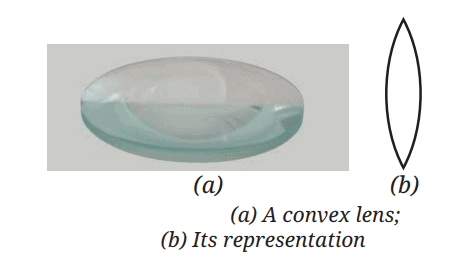
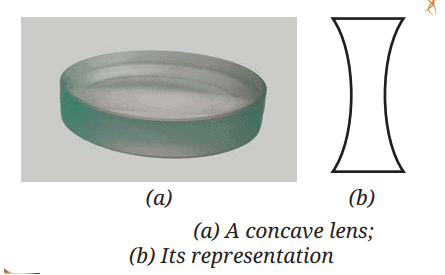
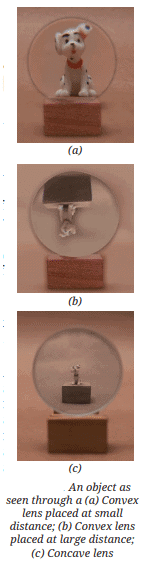
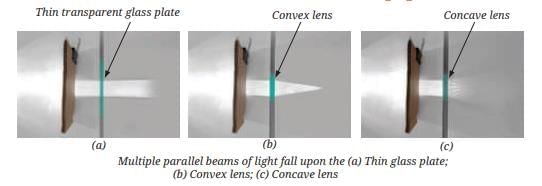
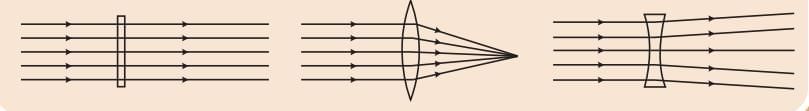

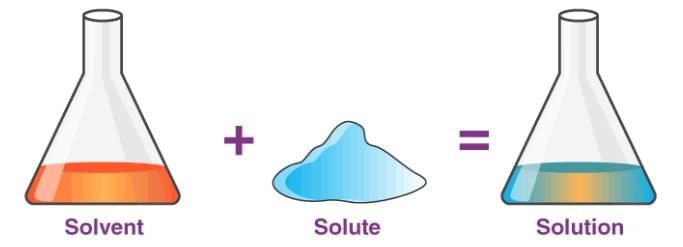
 Saturated Solution: A solution where no more solute can dissolve at a particular temperature, and excess solute settles at the bottom.
Saturated Solution: A solution where no more solute can dissolve at a particular temperature, and excess solute settles at the bottom.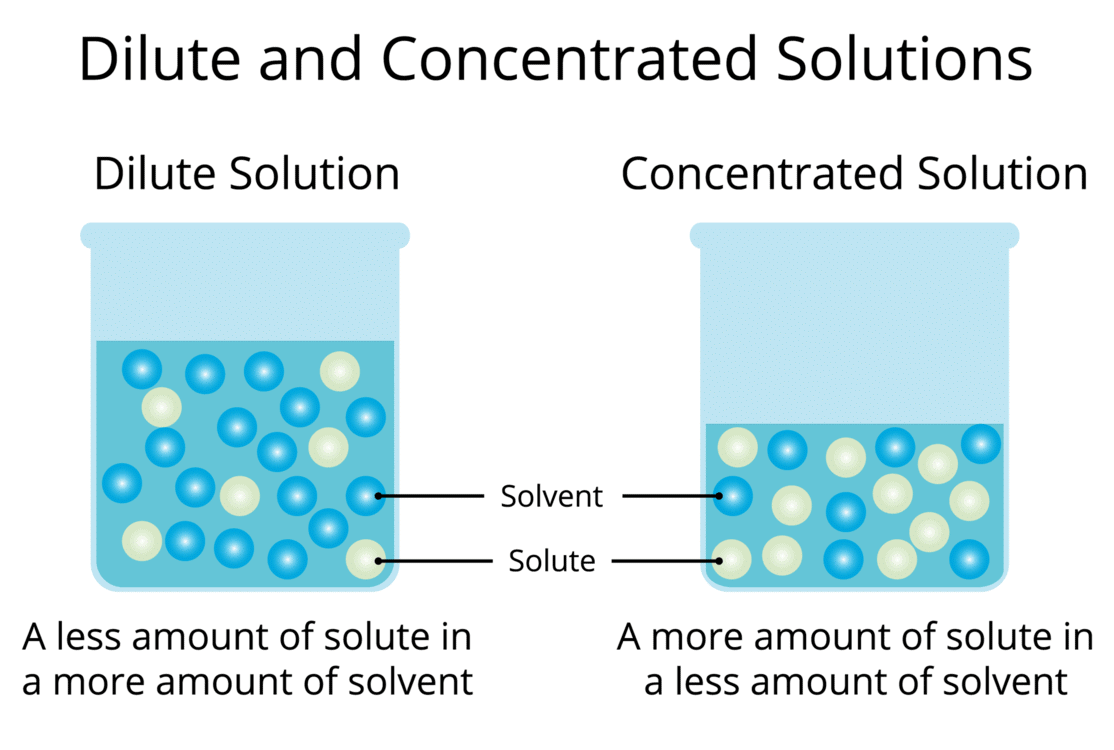 Concentrated Solution: A solution with a relatively large amount of solute in the solvent (e.g., more spoons of salt make it more concentrated).
Concentrated Solution: A solution with a relatively large amount of solute in the solvent (e.g., more spoons of salt make it more concentrated).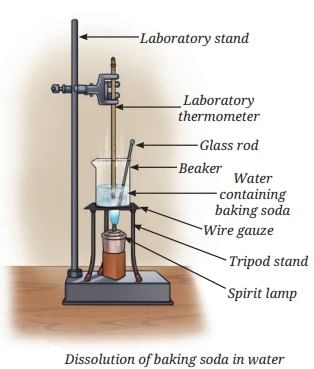


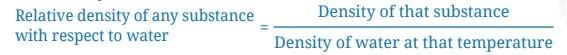




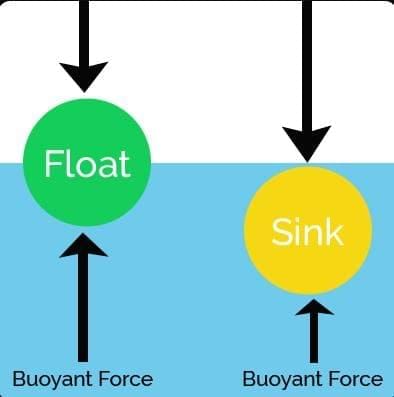
 Take a glass tumbler and fill it with tap water. Carefully place a raw whole egg into the water and observe what happens. You will notice that the egg sinks to the bottom.
Take a glass tumbler and fill it with tap water. Carefully place a raw whole egg into the water and observe what happens. You will notice that the egg sinks to the bottom.
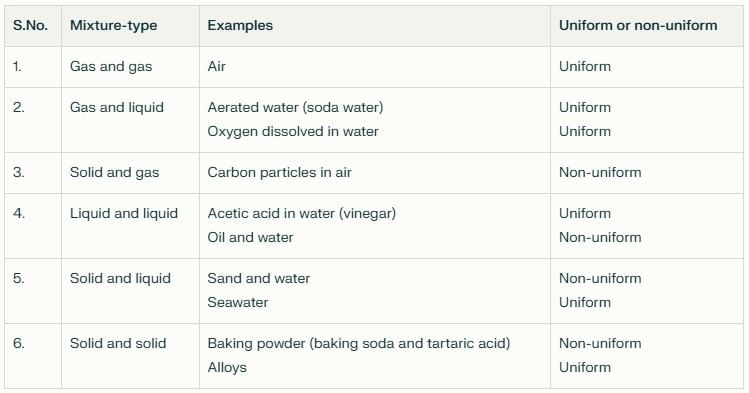
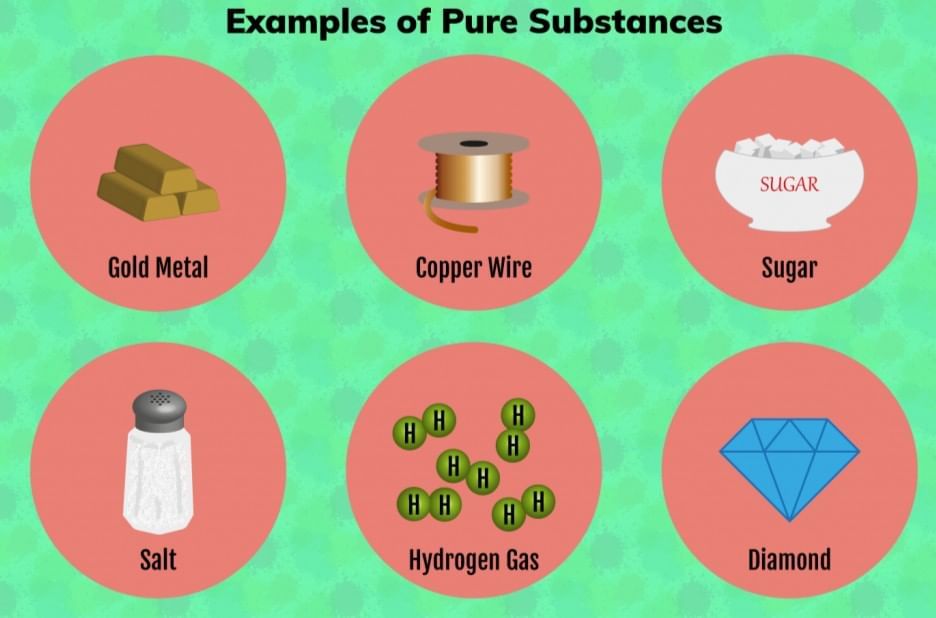
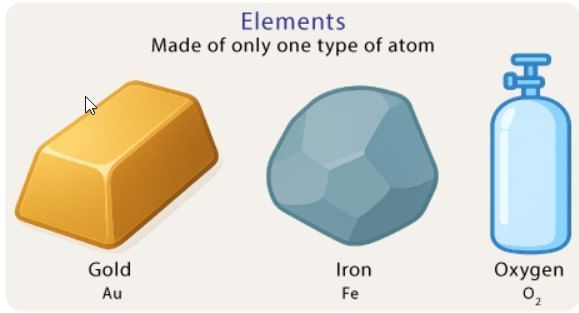
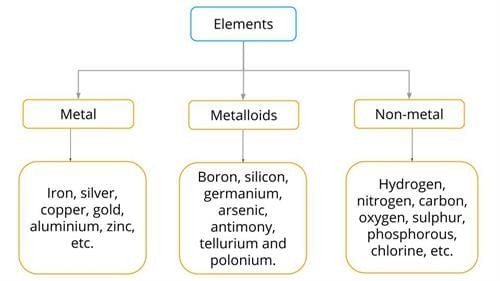






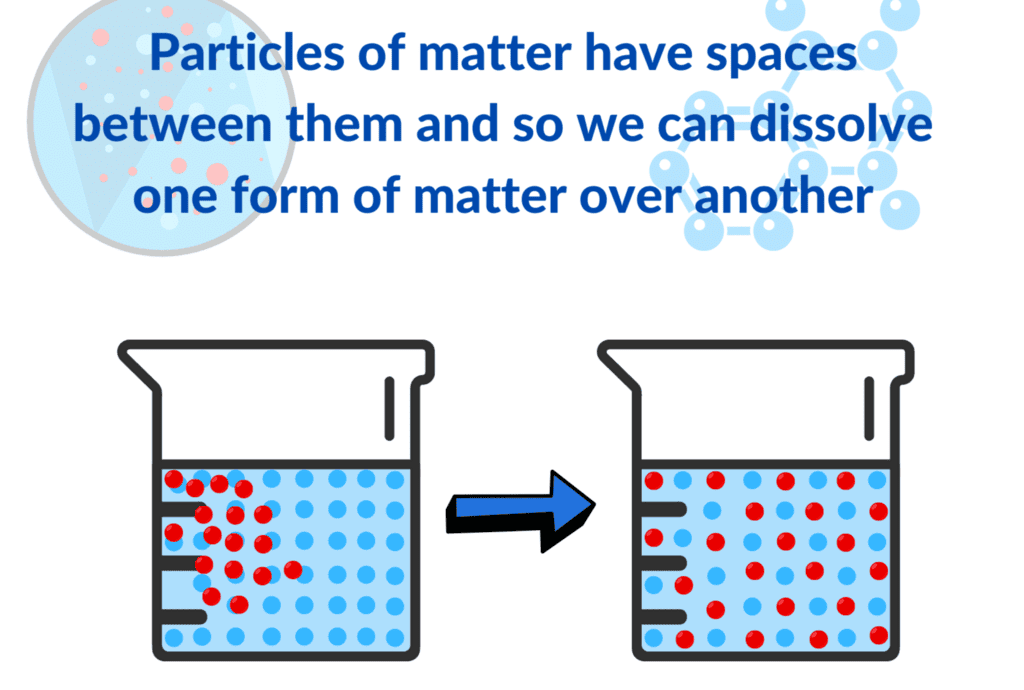
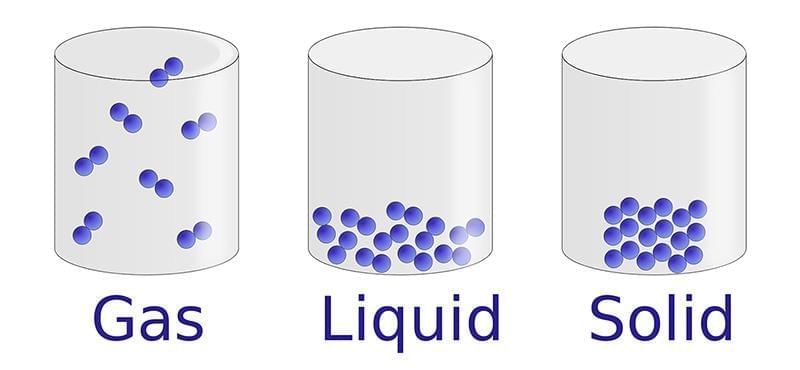

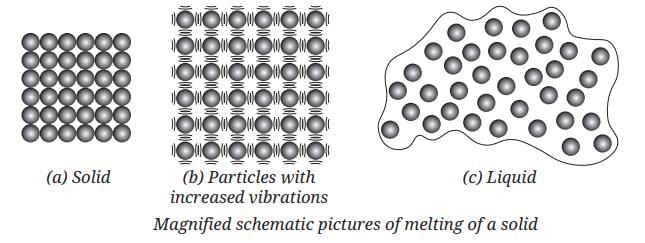
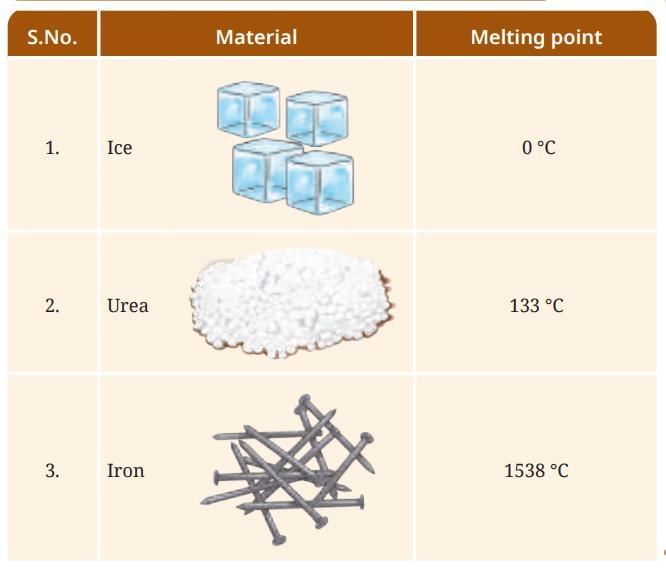
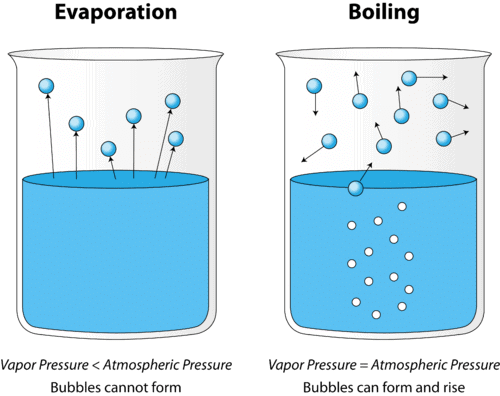 Evaporation: The slower process of vapor formation that occurs at all temperatures, even below the boiling point. Unlike boiling, it happens only at the liquid’s surface and proceeds gradually without bubble formation.
Evaporation: The slower process of vapor formation that occurs at all temperatures, even below the boiling point. Unlike boiling, it happens only at the liquid’s surface and proceeds gradually without bubble formation.
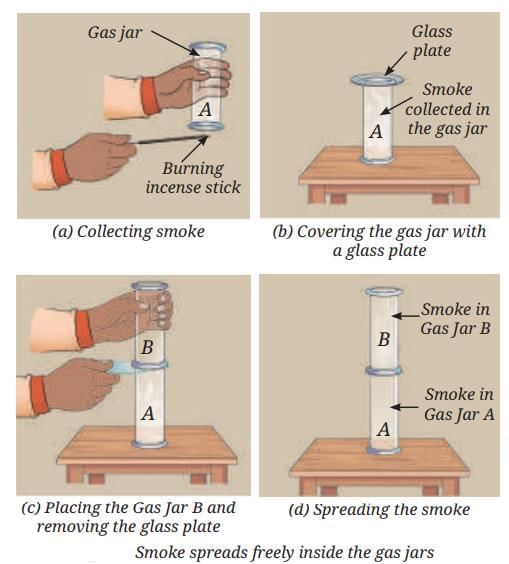

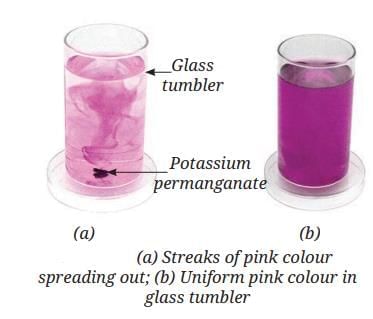
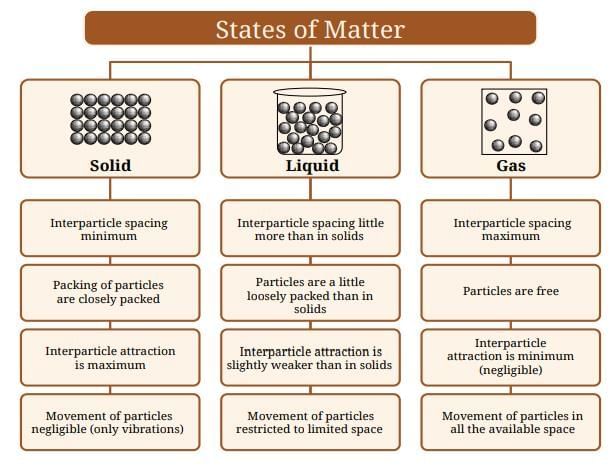


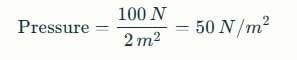
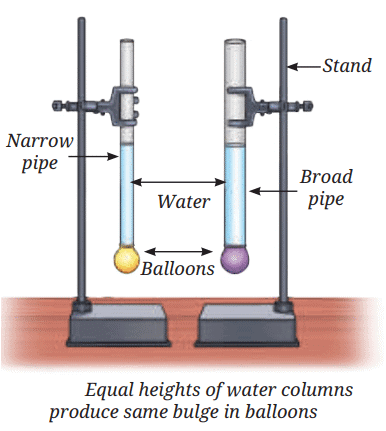
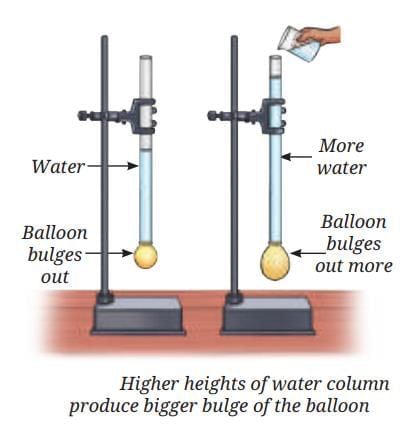
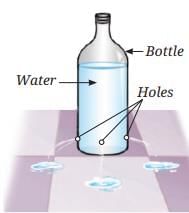
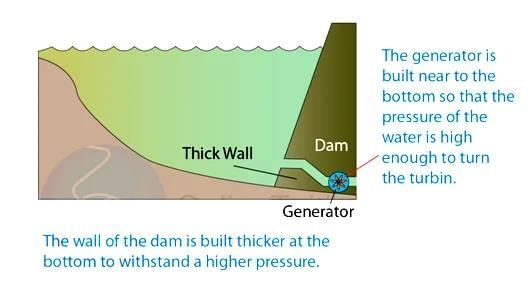
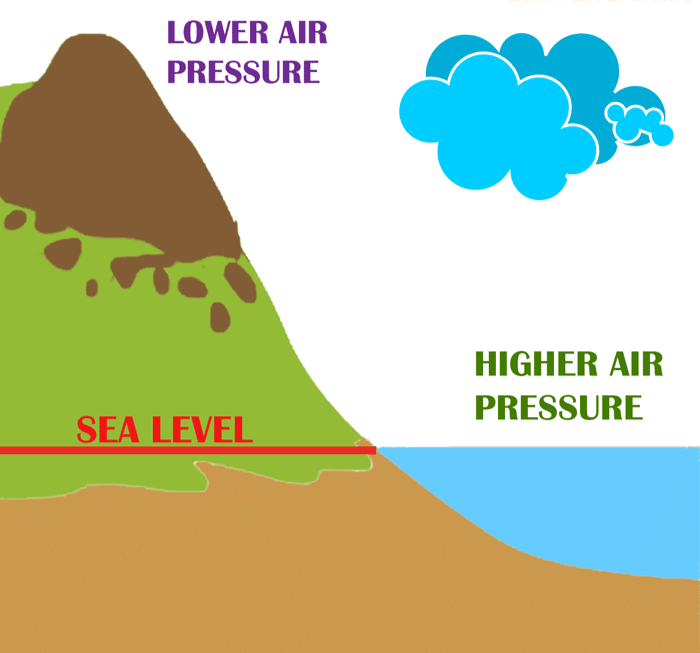
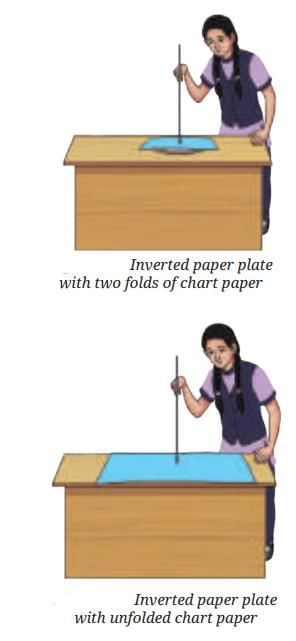
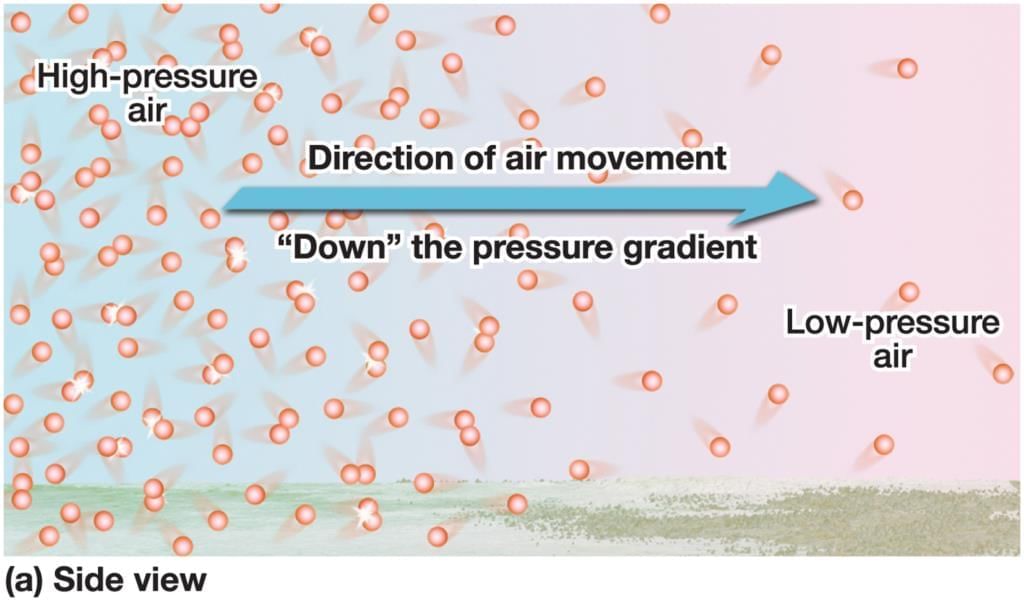
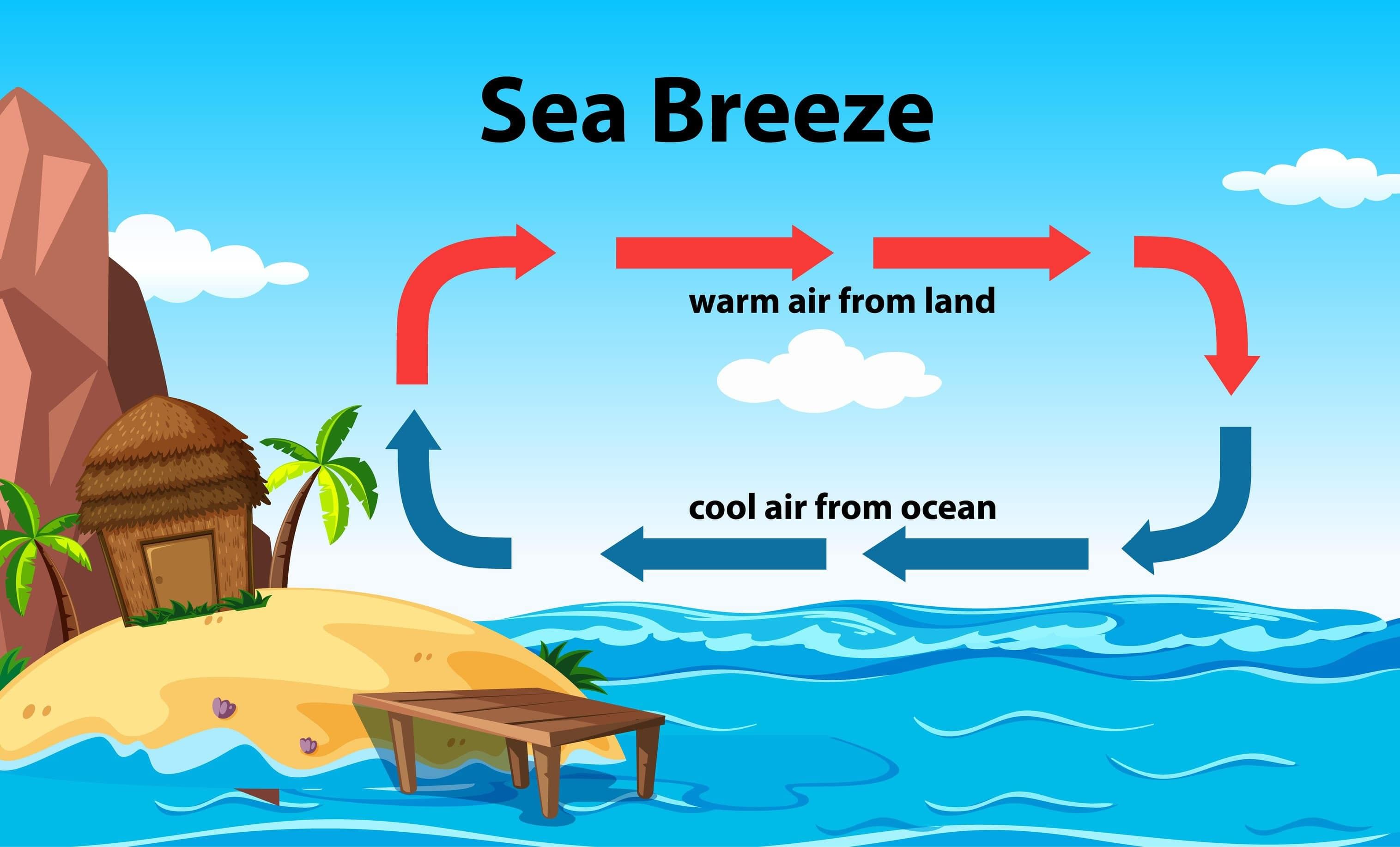 Sea Breeze
Sea Breeze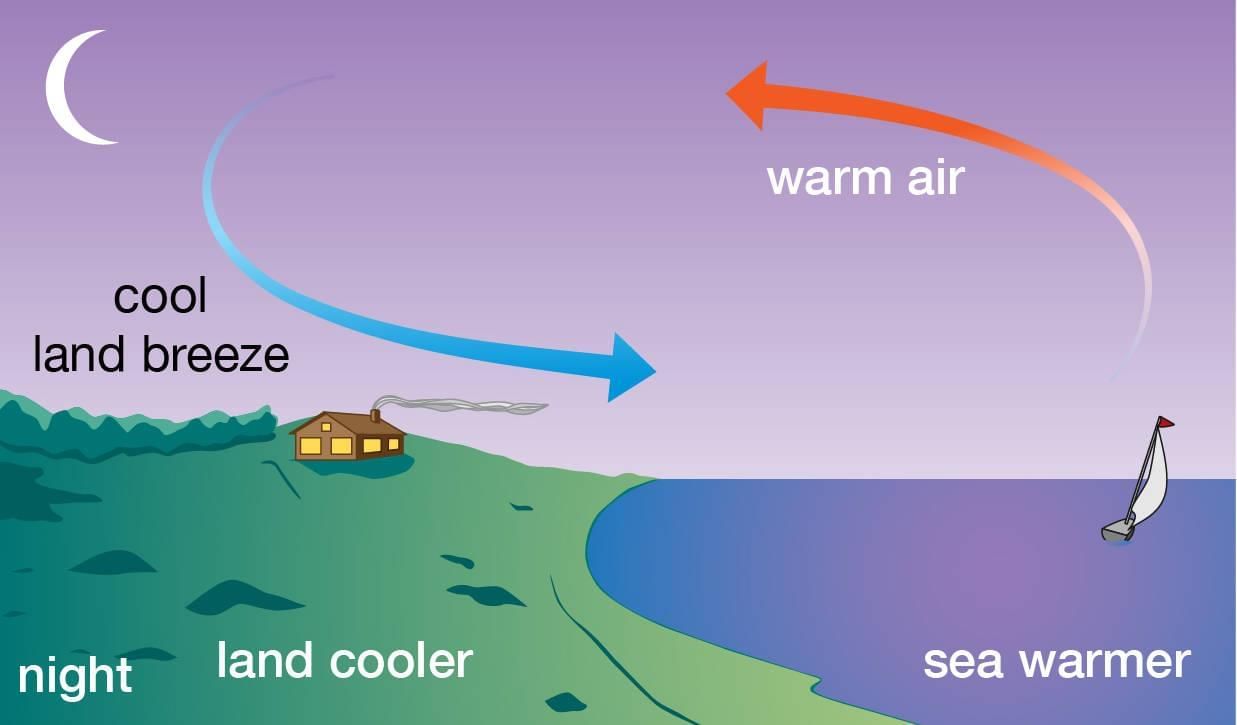 Land Breeze
Land Breeze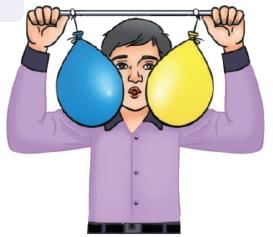 Blowing harder (faster air) makes them move even closer, more quickly.
Blowing harder (faster air) makes them move even closer, more quickly.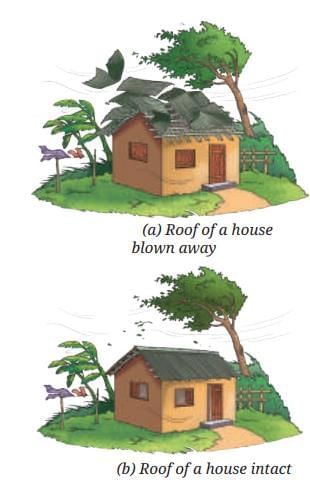

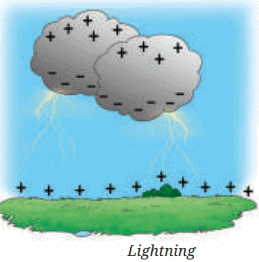 Heavier, negatively charged water droplets gather at the bottom.
Heavier, negatively charged water droplets gather at the bottom.


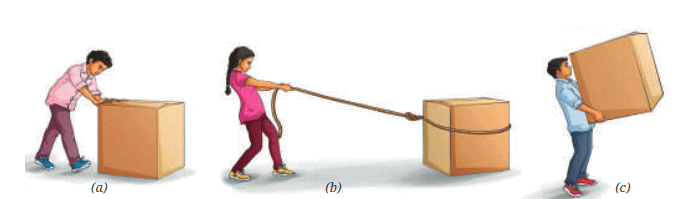
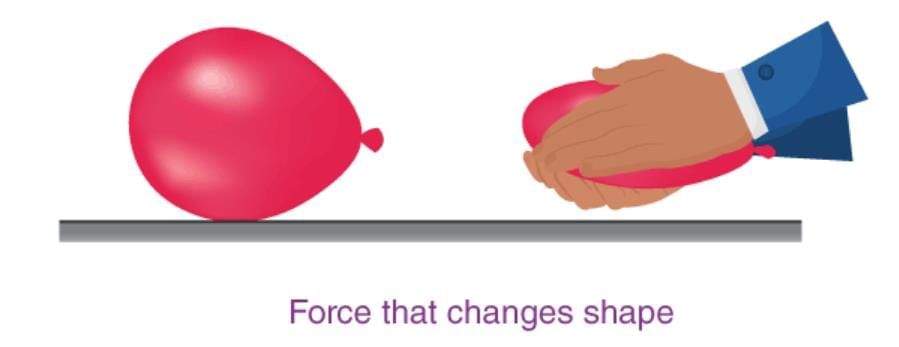
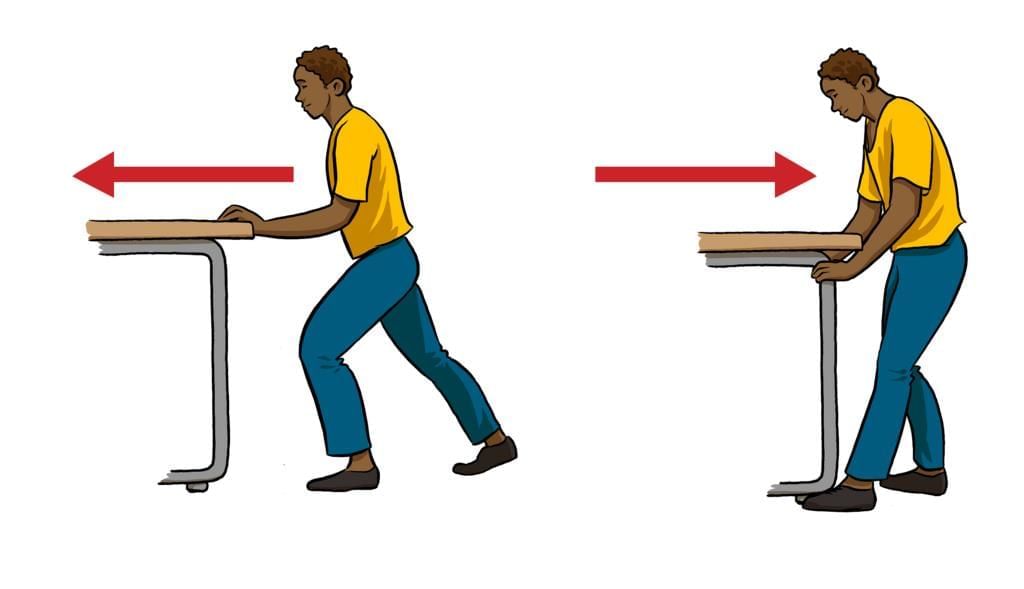
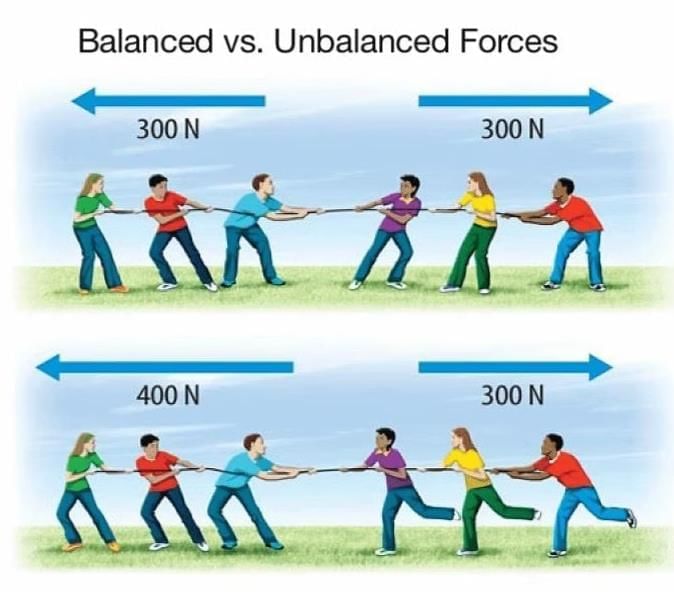

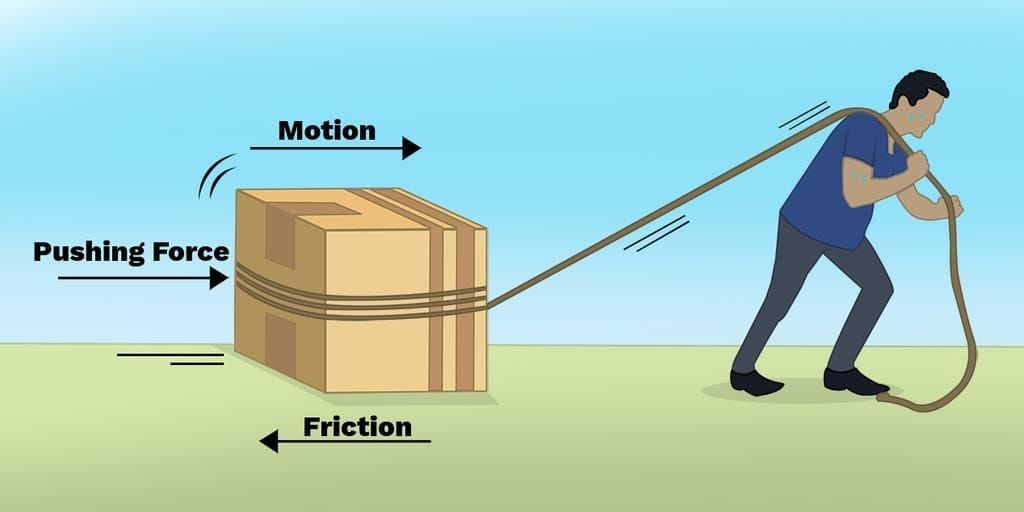
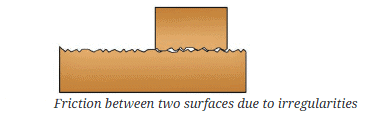
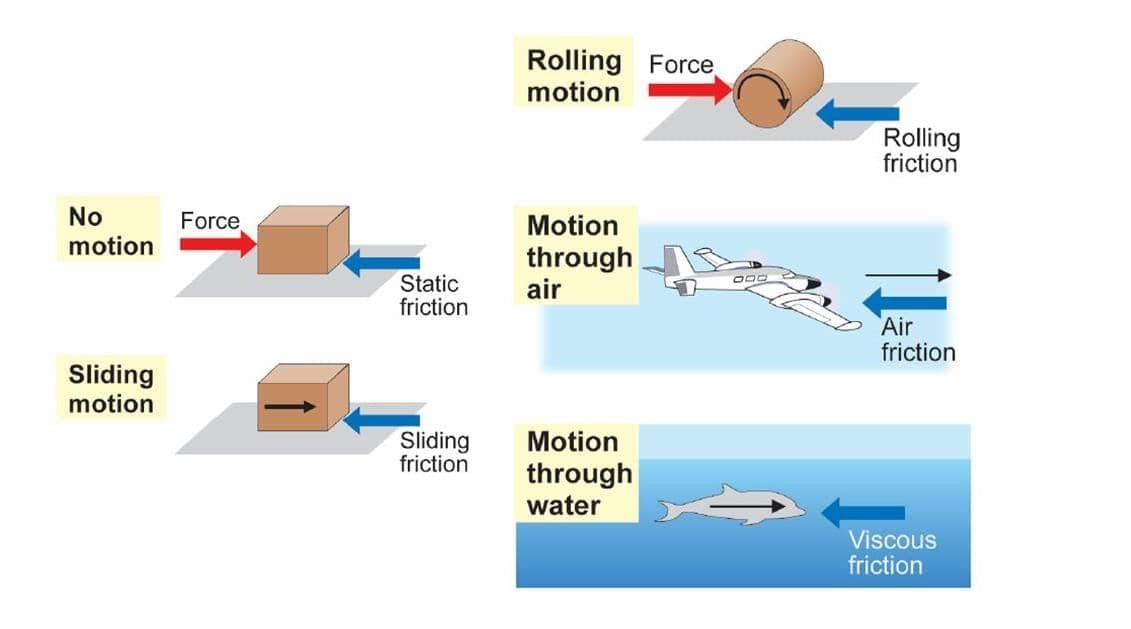
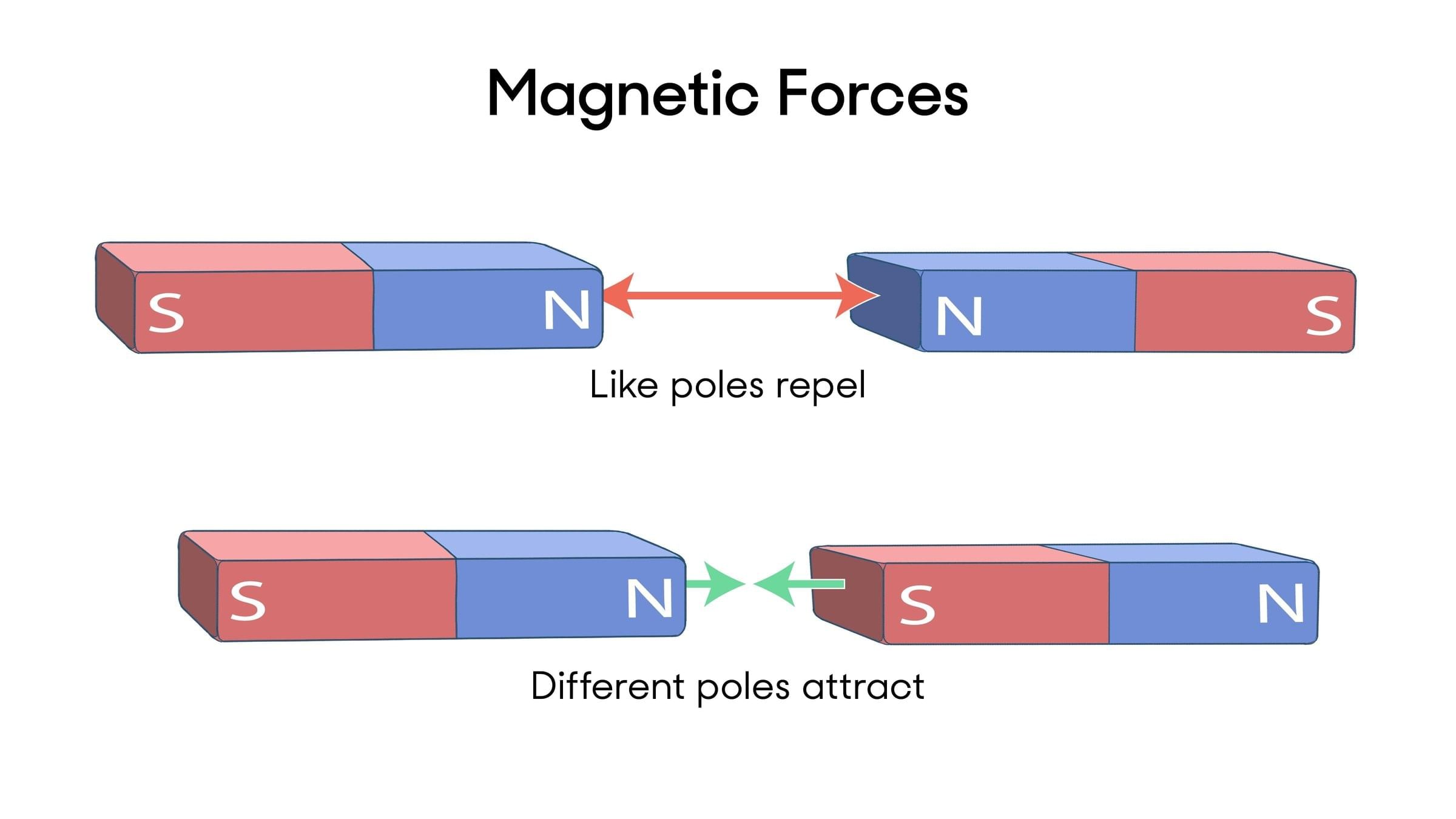

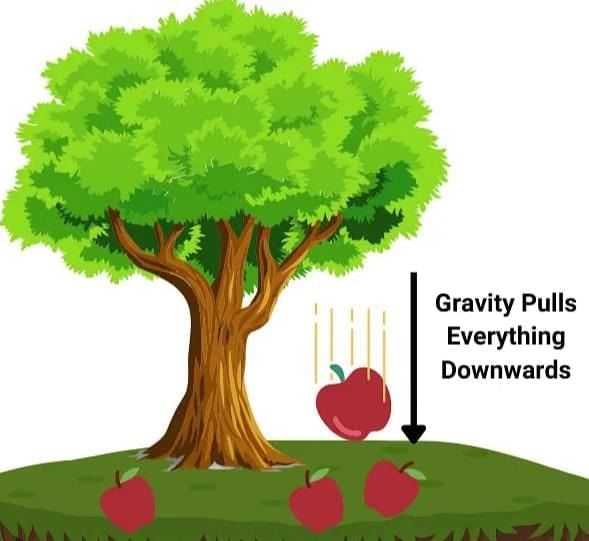
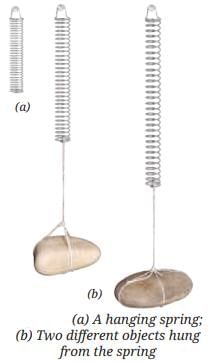
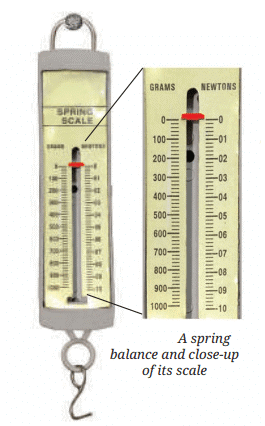
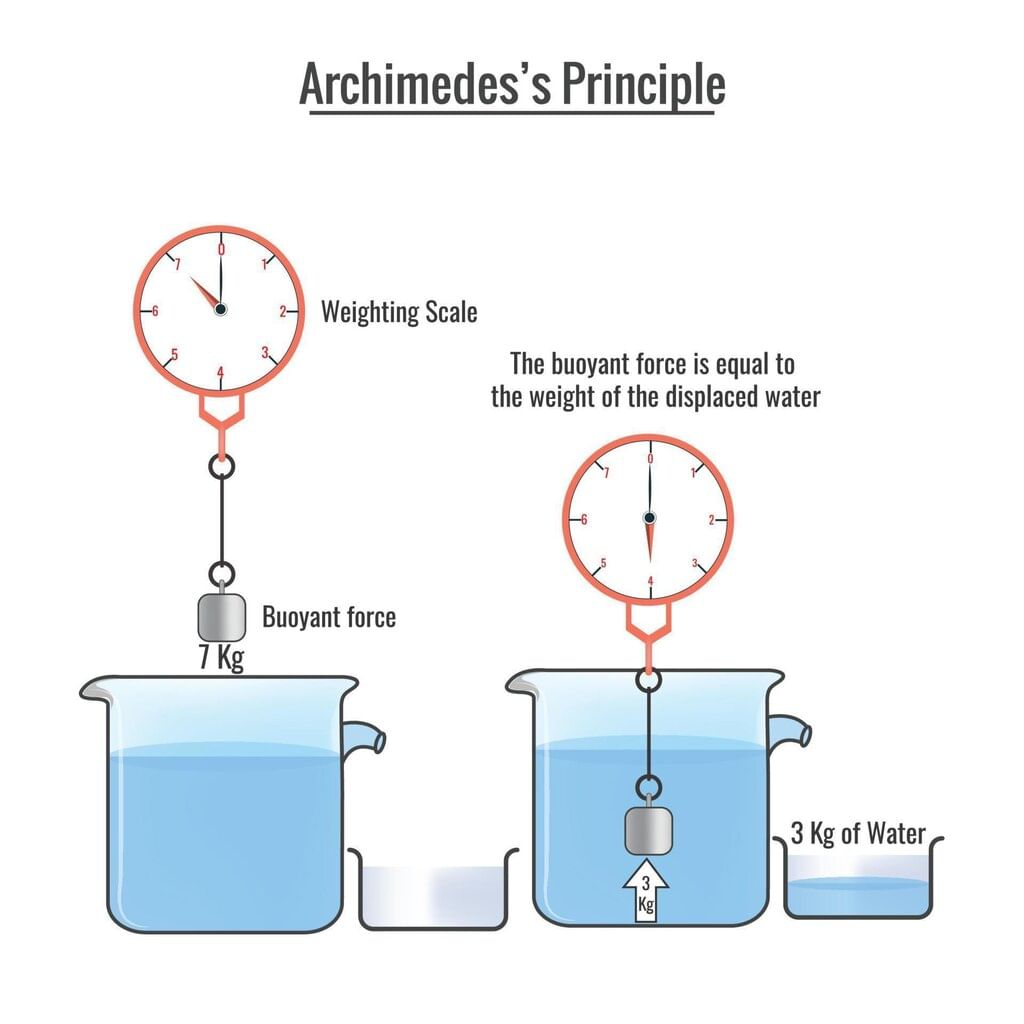
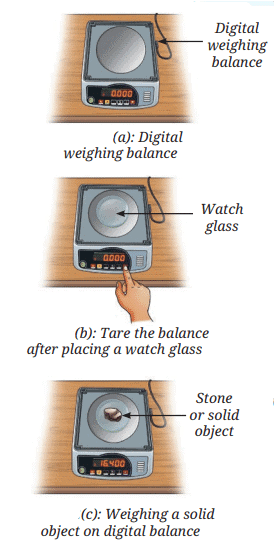
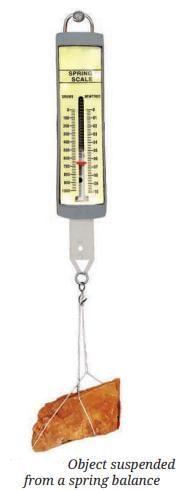 Measuring Weight with a Spring Balance: To measure the weight of an object, hang it from the hook of a spring balance (without exceeding its maximum range). The pointer or reading on the scale shows the object’s weight in newtons. This method can be repeated for many objects, and results should be recorded in a table.
Measuring Weight with a Spring Balance: To measure the weight of an object, hang it from the hook of a spring balance (without exceeding its maximum range). The pointer or reading on the scale shows the object’s weight in newtons. This method can be repeated for many objects, and results should be recorded in a table.