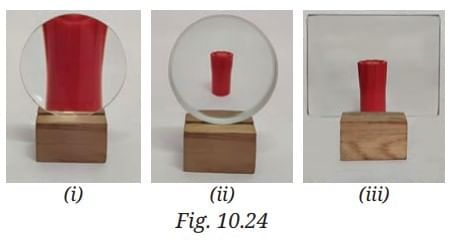
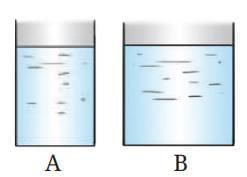
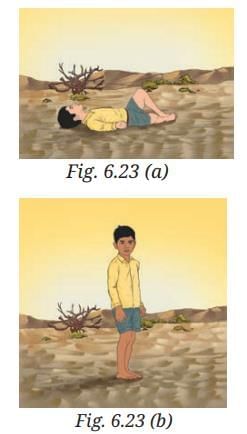
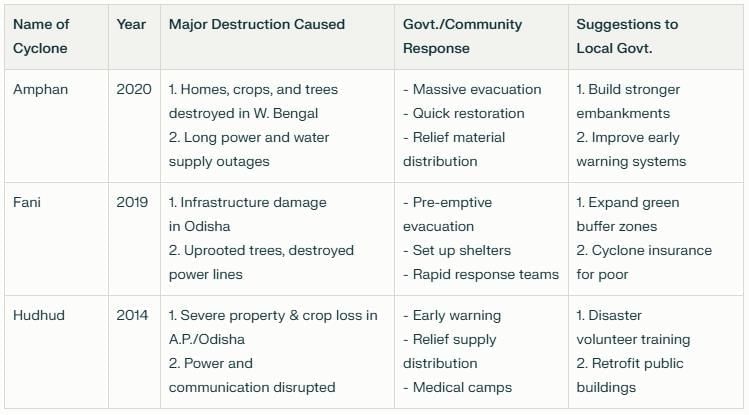
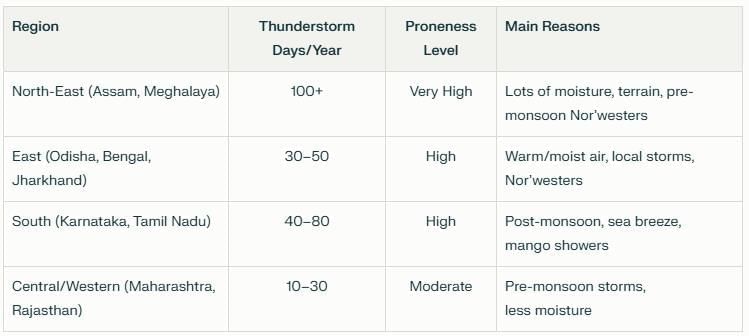
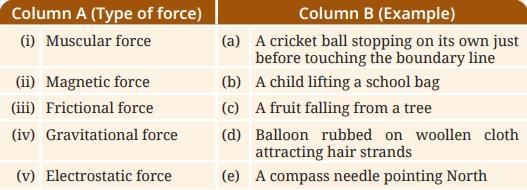
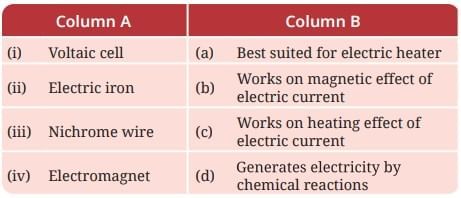
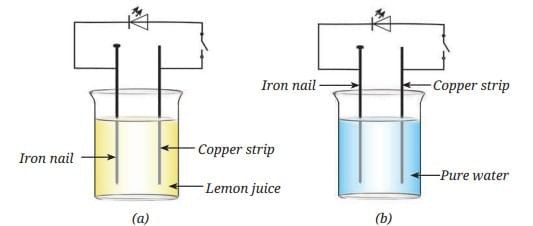
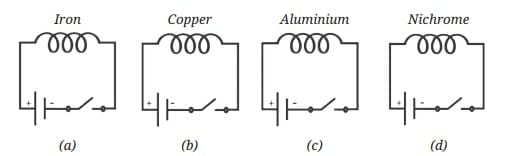
Probe and Ponder
- What do you think Earth would look like if there were no life on it at all?
Earth would look like a barren, rocky planet with vast empty lands, dry oceans, and no green forests or animals. There might be more dust storms, eroded mountains, and a thinner atmosphere without plants to produce oxygen or hold soil in place. It would be silent and lifeless, like Mars or the Moon. - Life on Earth has survived for billions of years. What allows it to keep going despite major changes and disasters?
Earth’s balance of air, water, sunlight, and soil, plus its position in the habitable zone, helps life adapt. Reproduction passes on traits that help survive changes, like disasters or climate shifts. Ecosystems recover through interactions between living things (like plants regrowing after a fire) and non-living parts (like nutrient recycling in soil). - Why don’t dogs lay eggs? Or hens give birth to live chicks?
Dogs are mammals, so they give birth to live young because their embryos develop inside the mother, getting nutrition directly. Hens are birds, so they lay eggs where the embryo grows outside with stored food. This is due to how each species evolved—mammals protect babies inside, while birds use eggs for safety in nests. - If a spaceship carried soil and water to Mars, could plants start growing there?
Maybe simple plants or microbes could grow if we add the right conditions, like protection from Mars’ thin atmosphere, extreme cold, and radiation. But most Earth plants would struggle without enough air pressure, liquid water stability, or Earth’s magnetic shield. Experiments show some bacteria survive, but full growth needs more, like a greenhouse dome. - Share your questions
Here are some fun questions from the chapter: Why is Earth called the Blue Planet? How does the ozone layer protect us? What would happen if Earth’s magnetic field disappeared? Could life exist on other planets in the habitable zone?
Keep the Curiosity Alive
- What is one major reason Mars cannot currently support life like Earth?
(i) It has too many volcanoes.
(ii) It is too close to the Sun.
(iii) It lacks a thick atmosphere and liquid water.
(iv) Its magnetic field is too strong.
Ans:
(iii) It lacks a thick atmosphere and liquid water.
Mars is at the edge of the habitable zone, but its thin atmosphere can’t trap heat or hold liquid water, making it too cold and dry for most life. - Which of these is an example of geodiversity?
(i) Variety of bird chirping in a forest.
(ii) Different landforms like mountains, valleys, and deserts.
(iii) Changing weather during monsoons.
(iv) Number of different types of fish in a pond.
Ans: (ii) Different landforms like mountains, valleys, and deserts.
Geodiversity is the variety of rocks, soils, and landforms that create unique habitats, supporting different plants and animals. - If the Earth were smaller with the same density, what might happen to its atmosphere?
(i) It would become thicker and hotter.
(ii) It would escape into space due to weaker gravity.
(iii) It would become frozen.
(iv) It would cause stronger winds.
Ans:
(ii) It would escape into space due to weaker gravity.
A smaller Earth couldn’t hold gases tightly, so the atmosphere would leak away, like on Mercury, leaving no air to breathe or protect life. - In sexual reproduction, why are offspring different from their parents?
(i) They grow in different climates.
(ii) They eat different food.
(iii) They acquire new instructions after birth.
(iv) They get mixed instructions (genes) from both parents.
Ans:
Gametes from each parent combine, mixing genetic material, so kids have traits from both but are unique, like varying eye colors in a family. - You notice tiny green plants growing in cracks on your school wall after the monsoon. Where do you think the seeds came from? What conditions helped these plants grow there?
Ans: Seeds likely came from wind, birds, or rain carrying them from nearby plants. Conditions like moisture from monsoon rain, trapped soil in cracks, and sunlight helped them germinate and grow roots—showing how plants adapt to tough spots. - A city has recently cut down a large patch of forest to build new roads and buildings. Discuss the possible effects this could have on the local climate and biodiversity? How might this affect water availability or quality in the area?
Ans: Local climate could get hotter and drier without trees to provide shade and release moisture. Biodiversity drops as animals lose homes, leading to fewer species. Water availability might decrease (less rain from reduced evaporation), and quality could worsen from soil erosion causing muddy runoff into rivers. - A friend says, “The Earth has always had climate changes in the past, so today’s global warming is nothing new.” How would you respond using what you’ve learnt in this and other chapters of your science book?
Ans: Past changes were natural and slow, like ice ages from Earth’s orbit shifts. Today’s warming is faster due to human actions like burning fossil fuels, releasing extra greenhouse gases. This causes extreme weather, rising seas, and biodiversity loss—unlike natural cycles. We need to act to reduce emissions. - Imagine Earth’s magnetic field suddenly disappeared. What kinds of problems could arise for life on Earth? Explain.
Ans: Harmful solar wind and cosmic rays would hit Earth directly, damaging the atmosphere, reducing ozone, and letting in more UV rays. This could cause more cancers, harm plants, and disrupt electronics. Life might struggle, especially in exposed areas, without this protective shield. - You are tasked with designing a new settlement for humans on Mars. Name three things you would need to recreate from Earth to support human life there. Which of these do you think is the hardest to replicate, and why?
Ans: Three things: Liquid water (for drinking and growing food), breathable atmosphere (with oxygen), and protection from radiation (like a magnetic field). Hardest is the atmosphere—Mars’ is thin and mostly carbon dioxide, so creating enough oxygen and pressure needs huge tech, unlike water which we could melt from ice. - In a village, the temperature has been increasing and rainfall has become unpredictable over the past few years. What could be causing this change? Suggest two ways the village could adapt to these new conditions.
Ans: Causes: Climate change from global warming, deforestation reducing local rain, or pollution trapping heat. Adapt by planting trees to cool the area and improve rain, and using rainwater harvesting to store water for dry times. - If there were no atmosphere on the Earth, would it affect life, temperature, and water on the planet? Explain.
Ans: Yes—without atmosphere, temperatures would swing wildly (hot days, freezing nights), water would evaporate or freeze easily, and no air means no breathing or wind. Harmful rays would reach the surface, killing most life. Atmosphere traps heat, holds water vapor, and shields us. - Discuss five examples of vegetative propagation.
Ans:
Potato: Eyes on tubers grow into new plants.
Ginger: Rhizomes (underground stems) sprout roots and shoots.
Money plant: Stem cuttings in water or soil grow roots.
Onion: Bulbs divide and form new plants.
Bryophyllum: Leaves with tiny plantlets fall and grow independently.
Discover, Design, and Debate
1. Design an ‘Earth Survival Kit’. Imagine you’re building a tiny model of Earth for another planet. What must it have to support life, and why?
Ans: My kit would include:
1. Liquid water (essential for cells, plants, and drinking—keeps things hydrated).
2. Breathable air with oxygen and carbon dioxide (for respiration and photosynthesis).
3. Soil with nutrients (to grow plants and recycle waste).
4. Sunlight simulator (for energy and warmth).
5. Magnetic shield model (to block harmful rays).
These recreate Earth’s balance—water and air sustain life, soil supports growth, sunlight provides energy, and the shield protects. Build a model with jars, plants, and magnets to show it!
2. India is planning for a challenging lunar mission, Chandrayaan-4, which will bring back samples of soil from the Moon. If the Moon had water, could plants grow in that soil? Think of some experiment that could help you explore whether plant growth is possible on the Moon.
Ans: Plants might grow if we add water, but Moon soil (regolith) lacks nutrients and has sharp particles that could harm roots. Experiment: Mix Moon-like soil (use fine sand or volcanic ash) with Earth soil in pots. Add water and plant seeds like beans. Compare growth in pure “Moon” soil vs. mixed—track height, leaves, and roots over weeks. If nothing grows in pure regolith, it shows we need to add nutrients or protection for lunar farming!
3. Flowers are often brightly coloured and have a pleasant smell. How do you think these features help the plant reproduce?
Ans: Bright colors and sweet smells attract pollinators like bees, butterflies, or birds. They visit for nectar, picking up pollen (male gametes) and carrying it to other flowers for fertilization. This helps plants make seeds without moving—nature’s way of mixing genes for stronger offspring!
4. Why do animals like fish and frogs lay hundreds or even thousands of eggs at a time, while other animals lay only a few? What might be the advantages and disadvantages of laying so many eggs?
Ans: Fish and frogs lay many eggs because most get eaten by predators or don’t survive in water—it’s a numbers game to ensure some hatch. Advantages: Increases survival chances without parental care. Disadvantages: Wastes energy if most fail, and no protection means high loss. Birds lay fewer but care for them, improving each egg’s odds!
5. Birds like sparrows build nests and care for their eggs and chicks, while reptiles like snakes usually lay their eggs and leave them without protection. How might this difference in parental care affect the chances of survival for the young ones in each case?
Ans: Sparrow chicks have higher survival because parents feed, warm, and protect them from predators—leading to more reaching adulthood. Snake eggs are hidden but face risks like weather or animals eating them, so fewer survive. Bird care boosts success but needs more energy; snake strategy spreads risk but has higher loss.
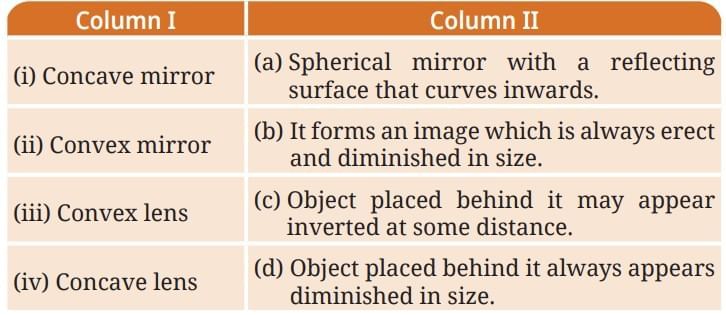
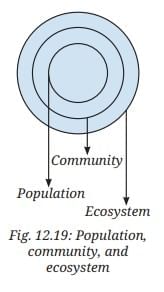 Refer to the given diagram (Fig. 12.19) and select the wrong statement.
Refer to the given diagram (Fig. 12.19) and select the wrong statement.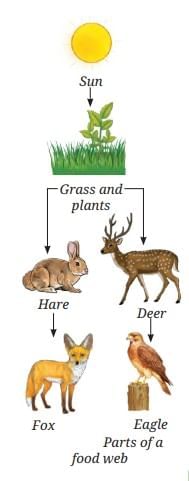 If the Indian hare population (Fig. 12.20) drops because of a disease, how would it affect the number of other organisms?
If the Indian hare population (Fig. 12.20) drops because of a disease, how would it affect the number of other organisms?