Multiple Choice Questions (MCQs)
Instruction: Select the correct option for each question.
- If Earth had never known life, which change in its atmosphere would most likely be true?
a) High oxygen from photosynthesis
b) Thick ozone layer formed naturally without oxygen
c) Very low oxygen and no biologically produced ozone
d) Same composition as today - Which surface feature would most likely be less abundant without life-driven soil formation?
a) Thick, organic-rich soils
b) Basaltic rocks
c) Impact craters
d) Volcanoes - In a lifeless Earth, which gas would most likely be higher due to unbalanced volcanic emissions?
a) Oxygen (O₂)
b) Carbon dioxide (CO₂)
c) Ozone (O₃)
d) Methane (CH₄) from microbes - Without plants and microbes, the long-term carbon cycle would be mainly controlled by:
a) Photosynthesis and respiration
b) Weathering, volcanism, and ocean chemistry
c) Pollination and seed dispersal
d) Decomposition by fungi - Which color would Earth most likely appear from space without life?
a) Deep blue with green land patches
b) Mostly brown/grey rocks with blue oceans and white clouds
c) Entirely green
d) Mostly white - On a lifeless Earth, the nitrogen cycle would be missing its main conversion by:
a) Lightning only
b) Microbes (nitrogen fixation/denitrification)
c) Volcanoes
d) Wind erosion - Which landform signal would likely be more preserved without life?
a) Rapid soil-covered slopes
b) Stable, sharp rock outcrops and more exposed craters
c) Dense root-bound riverbanks
d) Organic peat bogs - Absent life, which ocean characteristic would most likely differ?
a) Abundant dissolved oxygen from phytoplankton
b) Strong biological pump transporting carbon to deep ocean
c) No plankton blooms; chemistry set by abiotic processes
d) High biodiversity reefs - Which protective system would be weaker or absent without life?
a) Planetary magnetic field
b) Ozone layer sustained by O₂ from photosynthesis
c) Plate tectonics
d) Ocean tides - On a lifeless Earth, seasonal changes would mainly be due to:
a) Migration and leaf fall
b) Tilt-driven insolation patterns only
c) Flowering cycles
d) Plankton blooms
Fill in the Blanks
Instruction: Fill in the blanks with the correct word based on the chapter.
- Without photosynthesis, atmospheric __________ would remain very low and ozone would be weak.
- In a lifeless world, carbon would build up as __________ from volcanic emissions.
- Soils would be thin and largely mineral because there is no input of __________ matter.
- Ocean chemistry would lack a biological __________ that moves carbon to the deep sea.
- Lightning would be one of the few natural sources converting atmospheric nitrogen, in the absence of __________ fixation by microbes.
- Land surfaces would show more visible impact __________ without rapid biological weathering and soil cover.
- Without forests and phytoplankton, Earth’s surface would lack the green __________ of life.
- Climate would be set mainly by orbital position, volcanism, oceans, and the greenhouse effect, not by __________ feedbacks.
- The atmosphere, hydrosphere, geosphere, and magnetosphere would still interact, but the __________ would be missing.
- Satellite images would show bare rock, deserts, ice, clouds, and oceans, but no __________ patterns from crops or forests.
Very Short Answer Questions
Instruction: Answer the following questions in one line.
- What key atmospheric layer would be weaker without life?
- How would continents look from space without life?
- Which major biogeochemical cycles would be missing their main drivers?
- Would Earth still have plate tectonics without life?
- What would replace forests in shaping land surfaces?
Short Answer Questions
Instruction: Answer the following questions in 2–3 lines.
- Explain why oxygen would be scarce on a lifeless Earth.
- How would oceans differ without marine life?
- What visual signatures used by satellites to track ecosystems would disappear?
- Would weather and climate still vary in a lifeless world?
- How would the carbon cycle be balanced without photosynthesis and respiration?
Match the Following
Instruction: Match Column A with the correct option in Column B.
- Column A:
1) Ozone layer
2) Biological pump
3) Nitrogen fixation
4) Humus-rich soil
5) Green vegetation signal - Column B:
a) Satellite NDVI/green cover from plants
b) Deep-ocean carbon transport by plankton
c) Thicker, organic topsoil maintained by decomposers
d) UV shield formed from atmospheric O₂
e) Conversion of N₂ to usable forms by microbes
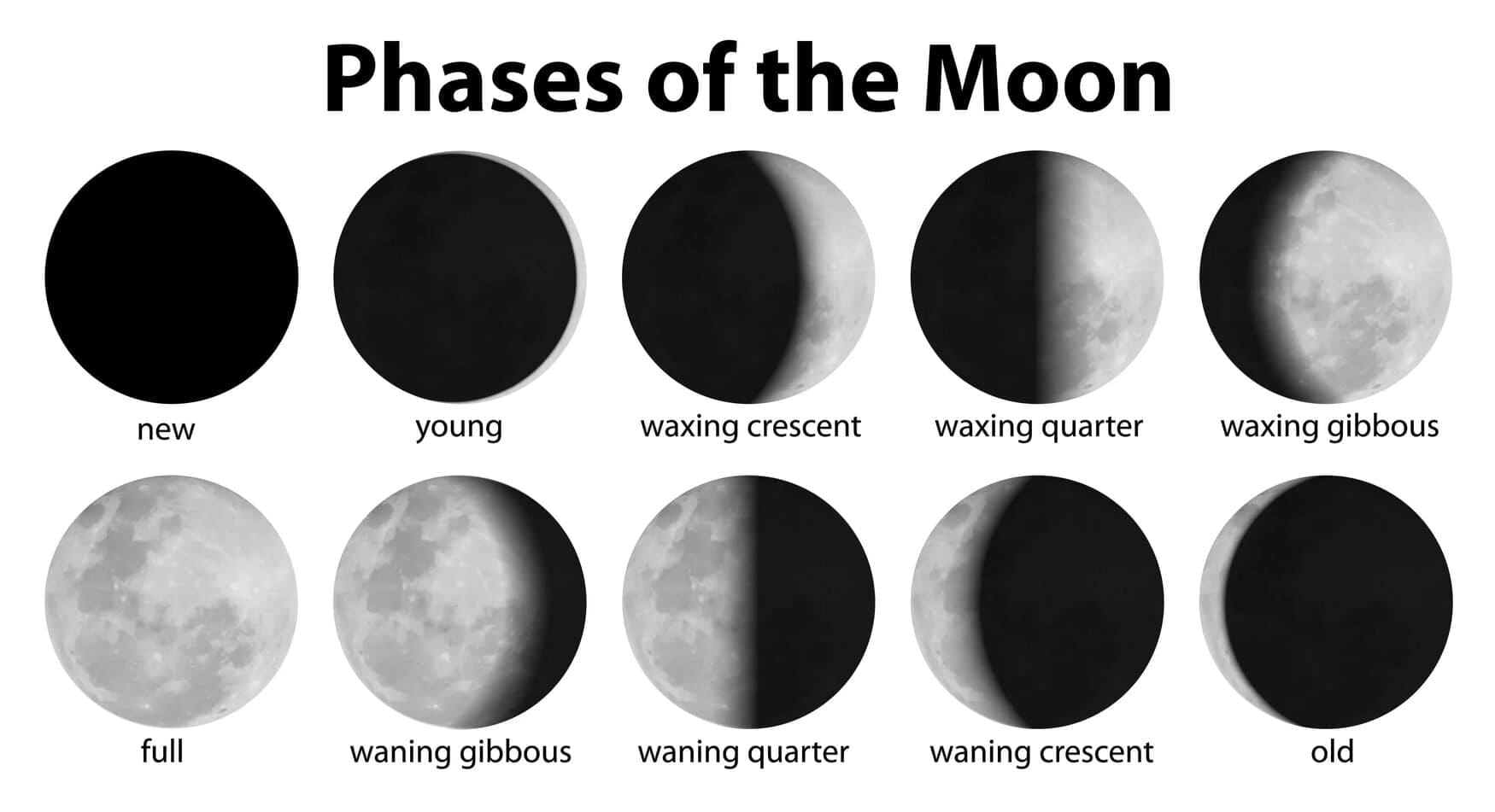 Why can we sometimes see the Moon in the daytime?
Why can we sometimes see the Moon in the daytime?



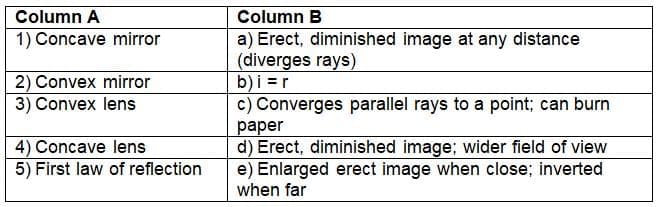
 Which mirror makes objects appear smaller but gives a wider field of view?
Which mirror makes objects appear smaller but gives a wider field of view?
 Which of the following forms a true (clear) solution with water?
Which of the following forms a true (clear) solution with water?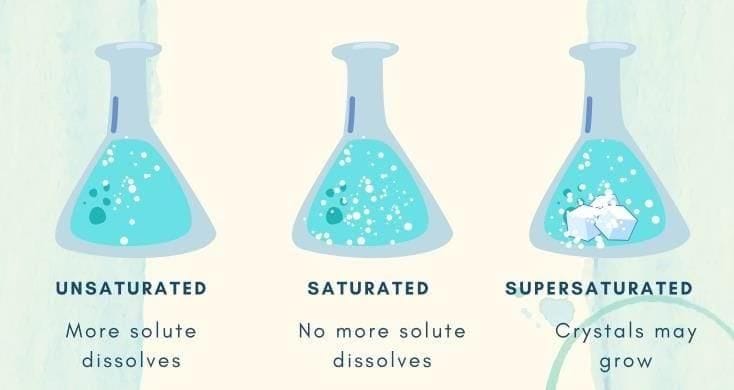 As more salt is added to a fixed amount of water, undissolved salt starts settling. The solution has become:
As more salt is added to a fixed amount of water, undissolved salt starts settling. The solution has become: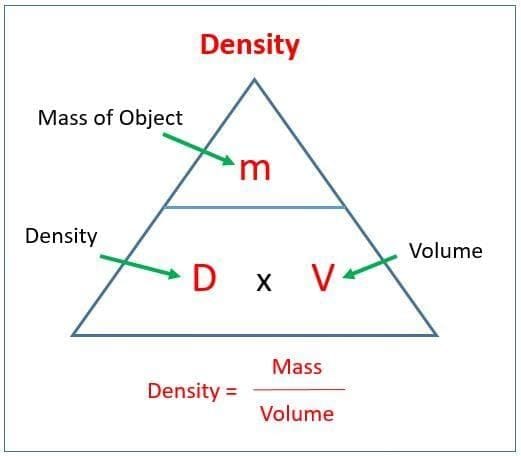




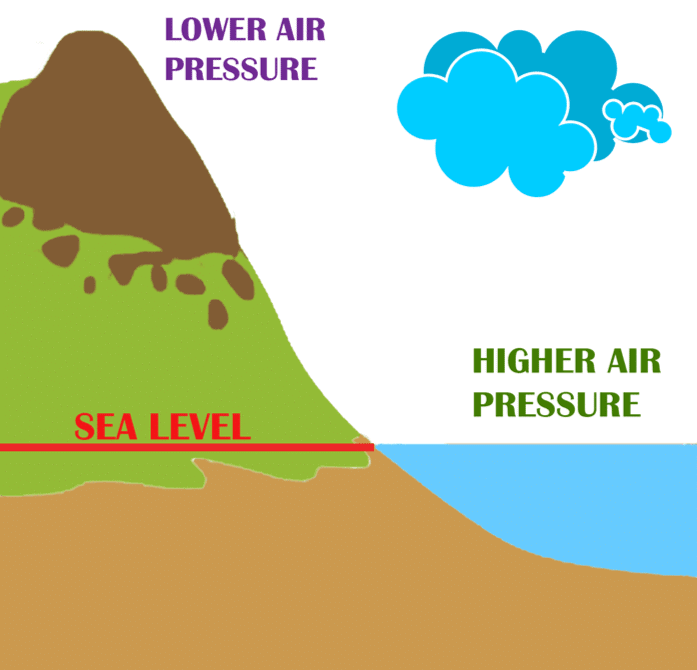 Atmospheric pressure is the pressure exerted by:
Atmospheric pressure is the pressure exerted by:
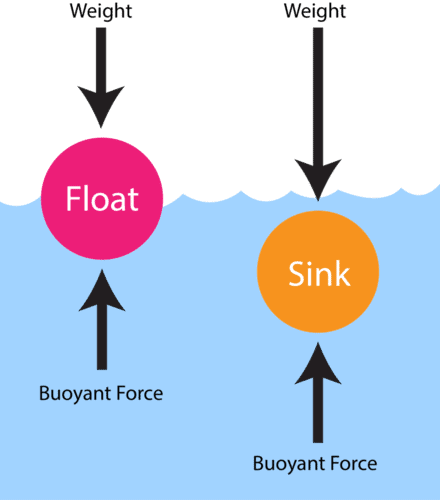
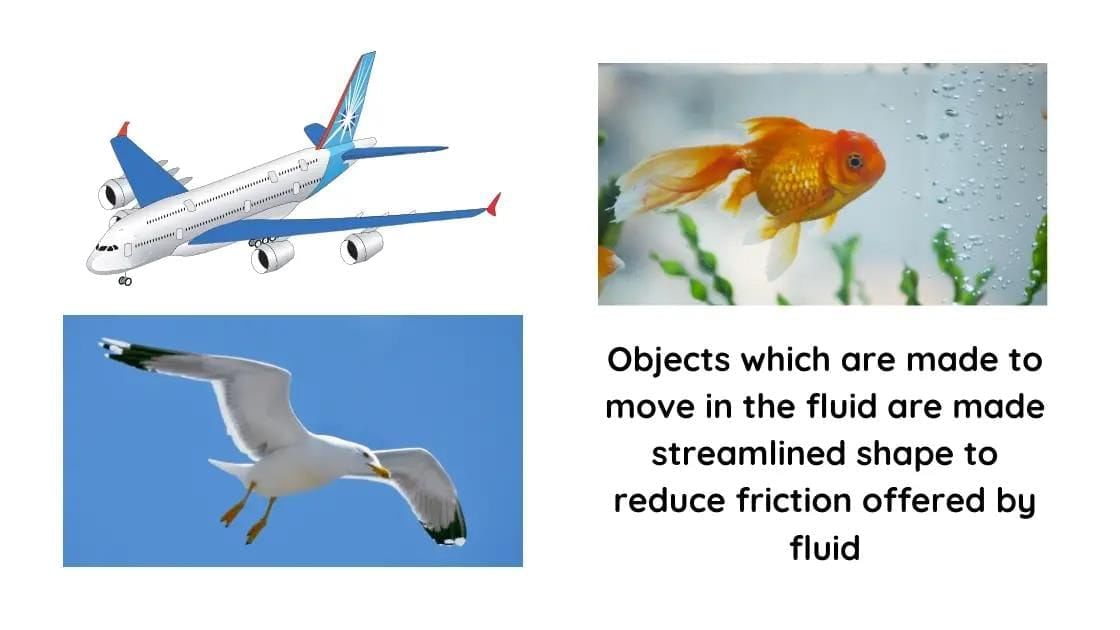

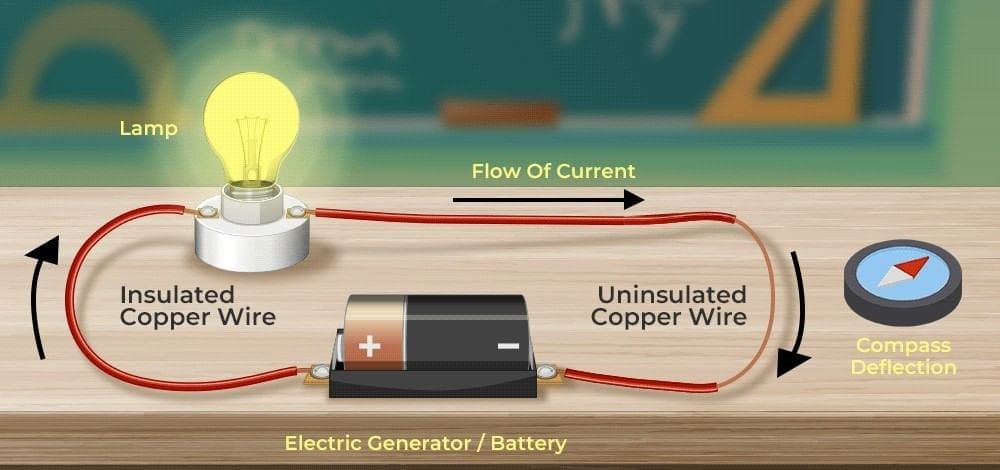 What did Oersted discover in 1820?
What did Oersted discover in 1820?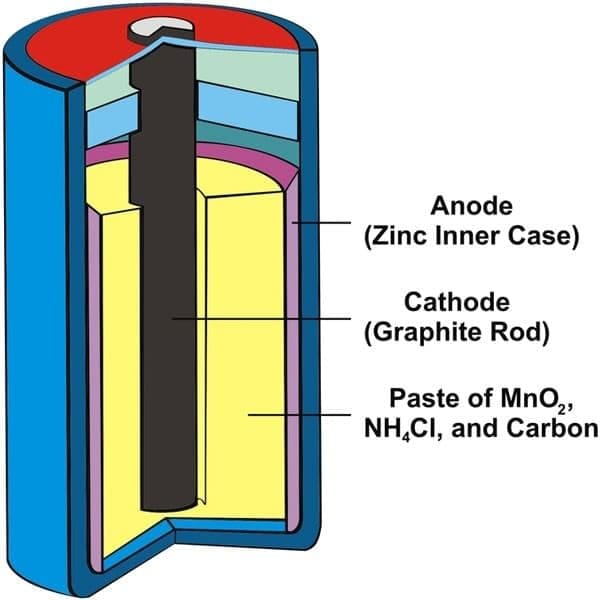 In a simple voltaic (galvanic) cell, electricity is produced by:
In a simple voltaic (galvanic) cell, electricity is produced by: