Previous Year Questions 2025
Q1: Electrolysis of water is a decomposition reaction. The mass ratio (MH : MO) of hydrogen and oxygen gases liberated at the electrodes during electrolysis of water is: (1 Mark)
(a) 8:1
(b) 2:1
(c) 1:2
(d) 1:8
Ans: (d) 1:8
During the electrolysis of water, water decomposes to form hydrogen and oxygen gases: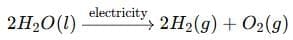
The volume ratio of hydrogen to oxygen is 2 : 1, but since oxygen is eight times heavier than hydrogen, the mass ratio (Mₕ : Mₒ) is 1 : 8.
Q2: Consider the following reactions: (1 Mark)
(i) Dilute hydrochloric acid reacts with sodium hydroxide
(ii) Magnesium oxide reacts with dilute hydrochloric acid
(iii) Carbon dioxide reacts with sodium hydroxide
It is found that in each case:
(a) Salt and water is formed
(b) Neutral salts are formed
(c) Hydrogen gas is formed
(d) Acidic salts are formed
Ans: (a) Salt and water is formed
In all three reactions, an acid reacts with a base (or basic oxide) to produce salt and water, which is a neutralisation reaction — a type of double displacement reaction.
Q3: In which one of the following situations a chemical reaction does not occur? (1 Mark)
(a) Milk is left open at room temperature during the summer
(b) Grapes get fermented
(c) An iron nail is left exposed to humid atmosphere
(d) Melting of glaciers
Ans: (d) Melting of glaciers
Melting of glaciers is only a physical change — the state of water changes from solid (ice) to liquid, but no new substance is formed. Hence, no chemical reaction takes place.
Q4: The correct balanced chemical equation showing exothermic reaction in which natural gas burns in air is: (1 Mark)
(a) CH₄ + O₂ → CO₂ + 2H₂O
(b) CH₄ + 2O₂ → 2CO₂ + 2H₂O + Energy
(c) CH₄ + 2O₂ → CO₂ + 2H₂O
(d) CH₄ + 2O₂ → CO₂ + 2H₂O + Energy
Ans: (d) CH₄ + 2O₂ → CO₂ + 2H₂O + Energy
When natural gas (methane) burns in air, it combines with oxygen to form carbon dioxide and water, releasing heat energy. This makes it an exothermic reaction.
Q5: Consider the following chemical equation: (1 Mark)
pAl + qH₂O → rAl₂O₃ + sH₂
To balance this equation, the values of ‘p’, ‘q’, ‘r’, and ‘s’ are:
(a) 3, 2, 2, 1
(b) 2, 3, 3, 1
(c) 2, 3, 1, 3
(d) 3, 1, 2, 2
Ans: (c) 2, 3, 1, 3
- Write skeletal equation: Al + H₂O → Al₂O₃ + H₂
- Balance Al → 2Al + H₂O → Al₂O₃ + H₂
- Balance O → 3H₂O → Al₂O₃ + H₂
- Balance H → 3H₂O → Al₂O₃ + 3H₂
Final: 2Al + 3H₂O → Al₂O₃ + 3H₂
Q6: The main observations while burning magnesium ribbon in air are: (1 Mark)
(i) Magnesium ribbon burns with a dazzling white flame
(ii) A white powder is formed
(iii) Magnesium ribbon vaporises
(iv) Aqueous solution of the white powder turns blue litmus red
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Ans: (c) (i) and (ii)
When magnesium ribbon burns in air, it burns with a dazzling white flame and forms a white powder of magnesium oxide, showing that a chemical reaction has taken place.
Q7: The values of a, b, c and d in the following balanced chemical equation are respectively: (1 Mark)
aPb(NO₃)₂ → bPbO + cNO₂ + dO₂
are:
(a) 1, 1, 2, 1
(b) 1, 1, 1, 2
(c) 2, 2, 1, 4
(d) 2, 2, 4, 1
Ans: (d) 2, 2, 4, 1
Balancing Steps (1 Mark):
- Write skeletal equation: Pb(NO₃)₂ → PbO + NO₂ + O₂
- Balance Pb: 1 atom on each side → no change.
- Balance N: There are 2 nitrogen atoms in Pb(NO₃)₂, so write 2Pb(NO₃)₂ → 2PbO + 4NO₂ + O₂.
- Balance O: Left side has 12 oxygen atoms; right side has 4 (in PbO) + 8 (in NO₂) = 12 → balanced.
Final balanced equation: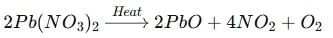
Q8: Examples of thermal decomposition reactions are: (1 Mark)
(i) 2AgCl → 2Ag + Cl₂
(ii) CaCO₃ → CaO + CO₂
(iii) 2H₂O → 2H₂ + O₂
(iv) 2KClO₃ → 2KCl + 3O₂
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (ii) and (iv)
Ans: (d) (ii) and (iv)
Thermal decomposition reactions are those that require heat to break down a compound into simpler substances.
- CaCO₃ → CaO + CO₂ (by heating)
- 2KClO₃ → 2KCl + 3O₂ (by heating)
Both are thermal decomposition reactions.
Q9: Assertion (a): Decomposition reactions are generally endothermic reactions. (1 Mark)
Reason (R): Decomposition of organic matter into compost is an exothermic process.
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, but R is false
(d) A is false, but R is true
Ans: (b) Both A and R are true, but R is not the correct explanation of A
- A: Decomposition reactions usually require energy (heat, light, or electricity), making them endothermic.
- R: Composting involves microbial breakdown, releasing heat (exothermic).
Both are true, but R describes an exception (biological process), not a general explanation for A.
Q10: Assertion (a): Silver chloride turns grey in sunlight. (1 Mark)
Reason (R): It decomposes into silver and chlorine in sunlight.
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, but R is false
(d) A is false, but R is true
Ans: (a) Both A and R are true and R is the correct explanation of A
When silver chloride is exposed to sunlight, it decomposes into silver and chlorine gas, turning the substance grey due to the formation of silver metal.
Q11: Assertion (a): All exothermic reactions are accompanied with evolution of heat and light. (1 Mark)
Reason (R): Combination reactions may or may not be exothermic.
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, but R is false
(d) A is false, but R is true
Ans: (d) A is false, but R is true
All exothermic reactions release heat, but not necessarily light.
Also, combination reactions may or may not be exothermic — for example, formation of slaked lime is exothermic, but not all combination reactions release light.
Q12: A student performs the following experiment in his school laboratory. List two observations to justify that in this experiment a chemical change has taken place. (2 Marks)
Ans:
- Bubbles of hydrogen gas are evolved when zinc granules react with dilute sulphuric acid.
- The test tube becomes warm, showing that heat is produced — an exothermic chemical reaction has taken place.
Reaction:
Zn + H2SO4 → ZnSO4 + H2
Q13: What happens when: (2 Marks)
(a) Lead nitrate is thermally decomposed
(b) Natural gas burns in air
(Write balanced chemical equations)
Ans:
(a) When lead nitrate is heated, it decomposes to form lead oxide, nitrogen dioxide, and oxygen.
Observation: Brown fumes of nitrogen dioxide are evolved.
(b) When natural gas (methane) burns in air, it forms carbon dioxide and water, releasing heat energy.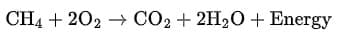
Observation: It is an exothermic reaction accompanied by the evolution of heat.
Q14: Translate and balance the following: (2 Marks)
(a) Nitric acid reacts with calcium hydroxide to form calcium nitrate and water
(b) Sodium chloride reacts with silver nitrate to form silver chloride and sodium nitrate
Ans:
(a) Word equation:
Nitric acid + Calcium hydroxide → Calcium nitrate + Water
Balanced chemical equation:
2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O
(b) Word equation:
Sodium chloride + Silver nitrate → Silver chloride + Sodium nitrate
Balanced chemical equation:
NaCl + AgNO3 → AgCl + NaNO3
Q15: Balance the following chemical equations: (2 Marks)
(a)
(b) 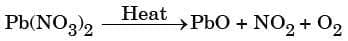
Ans:
(a) Photosynthesis reaction:
- Write formula: CO₂ + H₂O → C₆H₁₂O₆ + O₂.
- Balance C: glucose has 6 C → put 6 CO₂.
- Balance H: glucose has 12 H → put 6 H₂O.
- Check O: LHS O = 6×2 (from CO₂) + 6×1 (from H₂O) = 12+6 = 18. RHS O = 6 (in C₆H₁₂O₆) + 2×(coefficient of O₂). To get 18 total, O₂ coefficient must be 6 (6 + 12 = 18).
- Final balanced equation:
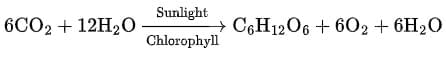
This is a balanced chemical equation representing the process of photosynthesis.
(b) Thermal decomposition of lead nitrate:
- Write products: Pb(NO₃)₂ → PbO + NO₂ + O₂.
- Start with 1 formula unit: Pb is balanced (1 Pb → 1 PbO). Nitrogen: 2 N on left → put 2 NO₂ on right.
- Count O with these coefficients: LHS O = 6 (from Pb(NO₃)₂). RHS O = 1 (in PbO) + 2×2 (in 2 NO₂) = 1 + 4 = 5 → missing 1 O atom. That comes from ½ O₂.
- To avoid fraction, multiply entire equation by 2.
- Final balanced equation:
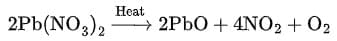
This is a balanced decomposition reaction with emission of brown fumes of NO₂.
Q16: What is observed when hydrated ferrous sulphate crystals are heated in a dry boiling tube? Write the balanced chemical equation. (2 Marks)
Ans:
When hydrated ferrous sulphate crystals (FeSO4·7H2O) are heated, they lose water and the green crystals turn white. On further heating, a brown solid (ferric oxide) and gases (sulphur dioxide and sulphur trioxide) are produced with a characteristic odour of burning sulphur.
Balanced chemical equation: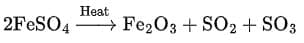
Q17: Write the balanced chemical equation for the reaction between aqueous solutions of sodium sulphate and barium chloride. State two types of reactions in which it is classified. (2 Marks)
Ans:
Equation: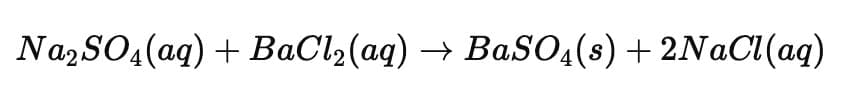
Types of reactions:
- Double displacement reaction – because there is an exchange of ions between reactants.
- Precipitation reaction – because an insoluble white precipitate of barium sulphate (BaSO₄) is formed.
Q18: A copper wire on burning gets coated with a black substance. (2 Marks)
(a) Write the chemical equation for the reaction
(b) How can this chemical change be reversed?
Ans: (a) Chemical equation:
Copper reacts with oxygen to form black copper(II) oxide.
(b) This change can be reversed by passing hydrogen gas over the heated black copper(II) oxide:
 The black coating turns brown again as copper metal is regenerated.
The black coating turns brown again as copper metal is regenerated.
Q19: (a) (i) Define the term decomposition reaction. Write one chemical equation each for decomposition reaction where energy is supplied in the form of heat, light or electricity.
(ii) Decomposition of vegetable matter into compost is considered an exothermic reaction. Why ?
OR
(b) Why are decomposition reactions called the opposite of combination reactions ? Write one chemical equation each for these two types of reactions mentioning the name of the reactant(s) and the product(s) involved in the reactions. (3 Marks)
Ans:
(i) A decomposition reaction is a reaction in which a single compound breaks down into two or more simpler substances when energy is supplied in the form of heat, light, or electricity.
Examples:
- By heat (thermal decomposition):
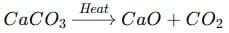
- By light (photodecomposition):
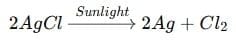
- By electricity (electrolytic decomposition):
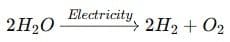
(ii) Decomposition of vegetable matter into compost is exothermic because it releases heat energy during the breakdown of organic matter.
OR
(b) Decomposition reactions are called the opposite of combination reactions because:
- In a combination reaction, two or more substances combine to form a single product.
- In a decomposition reaction, a single substance breaks down into two or more products.
Examples:
- Combination reaction:
 (Hydrogen and oxygen combine to form water.)
(Hydrogen and oxygen combine to form water.) - Decomposition reaction:
 (Lead nitrate decomposes to form lead oxide, nitrogen dioxide, and oxygen.)
(Lead nitrate decomposes to form lead oxide, nitrogen dioxide, and oxygen.)
Q20: How is a double displacement reaction different from a displacement reaction? Explain giving example in the form of balanced chemical equations. (3 Marks)
Ans:
Q21: What change is observed when copper powder is heated in a china dish? Name the phenomenon and write the balanced reaction. How is this different from the change observed when copper wares lose shine in moist air? Name the coating and its colour. (3 Marks)
Ans: When copper powder is heated in a china dish, its shiny brown surface turns black because copper reacts with oxygen to form black copper(II) oxide. This phenomenon is called oxidation.
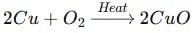
When copper wares lose their shine in moist air, they get coated with a green layer of basic copper carbonate, formed due to the reaction of copper with carbon dioxide and moisture present in air.
Thus, heating causes oxidation forming black CuO, whereas exposure to moist air forms a green coating of basic copper carbonate on copper.
Q22: (a) Why do we balance a chemical equation? Name and state the law that suggests the balancing of a chemical equation? Balance the following chemical equation: (3 Marks)
Zn + H₃PO₄ → Zn₃(PO₄)₂ + H₂
OR
(b) Define a precipitation reaction. Give its example and also express the reaction that occurs in the form of a balanced chemical equation. (3 Marks)
Ans:
(a) A chemical equation is balanced to ensure that the number of atoms of each element is the same on both sides of the equation, satisfying the Law of Conservation of Mass.
Law of Conservation of Mass: Mass can neither be created nor destroyed in a chemical reaction; the total mass of reactants equals the total mass of products.
Balancing the equation:
Unbalanced:
Zn + H3PO4 → Zn3(PO4)2 + H2
Balanced:
3Zn + 2H3PO4 → Zn3(PO4)2 + 3H2
OR
(b) A precipitation reaction is a double displacement reaction in which an insoluble solid (precipitate) is formed when two aqueous solutions react.
Example:
When sodium sulphate reacts with barium chloride, a white precipitate of barium sulphate is formed.
Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq)
Here, BaSO4 is the precipitate formed.
Q23: Write balanced equations for the following: (3 Marks)
(a) Steam passed over red-hot iron
(b) Natural gas burns in air
(c) Glucose reacts with oxygen in cells
Ans:
(a) When steam is passed over red-hot iron, ferric oxide and hydrogen gas are formed:
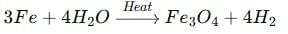
(b) When natural gas (methane) burns in air, it forms carbon dioxide and water, releasing heat energy:
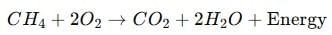
(c) When glucose reacts with oxygen in cells during respiration, it produces carbon dioxide, water, and energy:
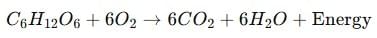
Q24: Explain why respiration is considered as an exothermic reaction. Give the chemical equation for this reaction. (3 Marks)
Ans:
Respiration is considered an exothermic reaction because during this process, energy is released in the form of heat when glucose reacts with oxygen in the cells of our body. This released energy is used by living organisms to perform various life functions.
Chemical equation:
Thus, respiration is an exothermic process as it liberates heat energy during the breakdown of glucose.
Previous Year Questions 2024
Q1: Select from the following a decomposition reaction in which the source of energy for decomposition is light: (2024)
(a) 2FeSO4 → Fe2O3 + SO2 + SO3
(b) 2H2O → 2H2 + O2
(c) 2AgBr → 2Ag + Br2
(d) CaCO3 → CaO + CO2
Ans: (c)
A decomposition reaction is when a compound breaks down into simpler substances. Option (c) 2AgBr → 2Ag + Br2 is the correct answer because it requires light energy to break down silver bromide (AgBr) into silver (Ag) and bromine (Br2). The other options do not use light for decomposition.
Q2: When 2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc in a test tube and then warmed, the reaction that occurs can be written in the form of a balanced chemical equation as: (2024)
(a) NaOH + Zn → NaZnO2 + H2O
(b) 2NaOH + Zn → Na2ZnO2 + H2
(c) 2NaOH + Zn → NaZnO2 + H2
(d) 2NaOH + Zn → Na2ZnO2 + H2O
Ans: (b)
When sodium hydroxide (NaOH) reacts with zinc (Zn), it produces sodium zincate and hydrogen gas. The balanced equation 2NaOH + Zn → Na2ZnO2 + H2 shows that two molecules of sodium hydroxide react with one piece of zinc to form sodium zincate and hydrogen gas. This is the correct representation of the reaction, making option (b) the right answer.
Q3: Select from the following a process in which a combination reaction is involved: (2024)
(a) Black and White photography
(b) Burning of coal
(c) Burning of methane
(d) Digestion of food
Ans: (b)
Burning of coal involves the reaction of carbon (coal) with oxygen to form carbon dioxide: C (s) + O2(g) → CO2(g). This is a combination reaction as two substances (carbon and oxygen) combine to form a single product (carbon dioxide).
Q4: Consider the following cases:
(A) CaSO4 + AI →
(B) CuSO4 + Ca →
(C) FeSO4 + Cu →
(D) ZnSO4 + Mg →
The cases in which new products will form are: (2024)
(a) (A) and (B)
(b) (B) and (C)
(c) (C)and(D)
(d) (B) and (D)
Ans: (d)
New products will form when a more reactive metal displaces a less reactive metal from its compound. In (B) and (D), calcium and magnesium are more reactive than copper and zinc, so they will successfully displace them, resulting in new products.
Q5: Identify the correct statement about the following reaction: (2024)
2H2S + SO2 → 2H2O + S
(a) H2S is oxidising agent and SO2 is reducing agent.
(b) H2S is reduced to sulphur.
(c) SO2 is oxidising agent and H2S is reducing agent.
(d) SO2 is oxidised to sulphur.
Ans: (c)
In the reaction, hydrogen sulfide (H2S) loses hydrogen to form sulfur (H2S is oxidized to sulfur), which means it acts as a reducing agent, while sulfur dioxide (SO2) gains hydrogen to form
H2O (SO2 is reduced to H2O), which means it acts as an oxidizing agent. Therefore, the correct statement is (c) because SO2 is the oxidizing agent and H2S is the reducing agent in this reaction.
Q6: Consider the following Chemical equation: (2024)
In order to balance this chemical equation, the values of a, b, c and d must be
(A) 1, 6, 2 and 3
(B) 1, 6, 3 and 2
(C) 2, 6, 2 and 3
(D) 2, 6, 3 and 2
Ans: (a)
To balance the chemical equation Al2O3 + HCl → AlCl3 + H2O , we need to ensure that the number of each type of atom is the same on both sides. The correct coefficients are 1 for Al2O3, 6 for HCl, 2 for AlCl3, and 3 for H2O, giving option (A) as the right choice. The balanced equation is 
Q7: Which of the following reactions is different from the remaining three? (2024)
(a) NaCl + AgNO3 → AgCl + NaNO3
(b) CaO + H2O → Ca(OH)2
(c) KNO3 + H2SO4 → KHSO4 + HNO3
(d) ZnCl2 + H2S → ZnS + 2HCl
Ans: (b)
In the reactions listed, options (a), (c), and (d) involve double displacement or exchange of ions between reactants, where compounds are formed by swapping partners. However, option (b) is a combination reaction, where calcium oxide (CaO) reacts with water (H2O) to form calcium hydroxide (Ca(OH)2) without exchanging ions. This makes (b) different from the others.
Q8: Zn + 2CH3COOH → Zn(CH3COO)2 + H2 (2024)
The above reaction is a:
(a) Decomposition reaction
(b) Displacement reaction
(c) Double displacement reaction
(d) Combination reaction
Ans: (b)
In the given reaction, zinc (Zn) replaces hydrogen in acetic acid (CH3COOH) to form zinc acetate (Zn(CH3COO)2) and hydrogen gas (H2). This type of reaction, where one element displaces another from a compound, is called a displacement reaction. Therefore, option (b) is correct.
Q9: To balance the following chemical equation, the values of the coefficients x, y and z must be respectively : (2024)
(a) 4, 2, 2
(b) 4, 4, 2
(c) 2, 2, 4
(d) 2, 4, 2
Ans: (c) x Zn(NO3)2 → y ZnO + z NO2 + O2
Step 1: Assume x = 2 (2 molecules of Zn(NO3)2).
Step 2:Distribute atoms:
- Zn: 2 atoms → 2 ZnO (y = 2)
- N: 4 atoms → 4 NO2 (z = 4)
- O: 12 atoms → 2 in ZnO, 8 in NO2, remaining 2 form O2.
Step 3:Verify all elements are balanced:
- Zn: 2 on both sides.
- N: 4 on both sides.
- O: 12 on both sides.
2Zn(NO3)2 → 2ZnO + 4NO2 + O2
Q10: Which of the following is a redox reaction, but not a combination reaction? (2024)
(a) C + O2 → CO2
(b) 2 H2 + O2 → 2 H2O
(c) 2 Mg + O2 → 2 MgO
(d) Fe2O3 + 3 CO → 2 Fe + 3 CO2
Ans: (d)
A redox reaction involves both oxidation and reduction, where electrons are transferred between substances. In option (d), iron(III) oxide (Fe2O3) is reduced to iron (Fe), while carbon monoxide (CO) is oxidized to carbon dioxide (CO2). Unlike the other options, which are combination reactions, this one shows the reduction and oxidation of different substances, making it a redox reaction but not a combination reaction.
Q11: Name the type of chemical reaction in which calcium oxide reacts with water. Justify your answer by giving a balanced chemical equation for the chemical reaction. (2024)
Ans: Combination reaction – Single product is formed (or any other)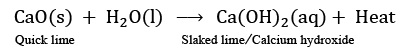
Q12: Write one chemical equation each for the chemical reaction in which the following have taken place:
(i) Change in colour
(ii) Change in temperature
(iii) Formation of precipitate
Mention colour change/temperature change (rise/fall)/compound precipitated along with the equation. (2024)
Ans: (i) Change in colour: The solution will become green in colour.
(ii) Change in temperature: The temperature will increase.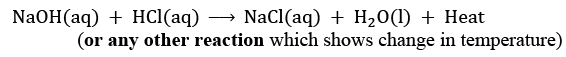
(iii) Formation of precipitate: Yellow precipitate of PbI2 is formed.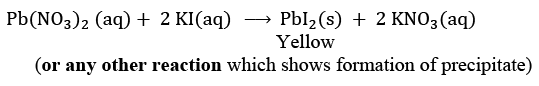
Q13: When magnesium ribbon is burnt in the air, an ash of white colour is produced. Write the chemical equation for the reaction giving the chemical name of the ash produced. State the type of chemical reaction justifying your answer. (2024)
Ans: 2 Mg + O2 → 2MgO
Magnesium oxide
Type – Combination reaction
Reason: Two or more substances combine to form a single product.
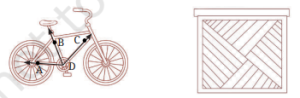
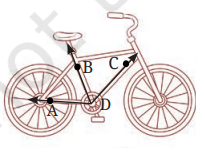
Q14:Study the experimental set-up shown in the diagram and write a chemical equation for the chemical reaction involved. Name and define the type of reaction. List two other metals that can be used in place of iron to show the same type of reaction with copper sulphate solution. (2024)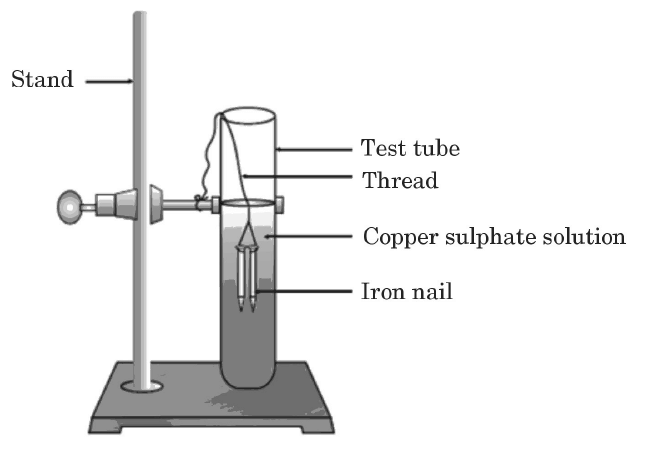
Ans: Fe(s) + CuSO4 (aq) → FeSO4(aq) + Cu(s)
Displacement reaction: A reaction in which a more reactive metal displaces a less reactive metal from its salt solution.
Other metals that can be used in place of iron to show the same type of reaction with copper sulphate solution: Zinc, Aluminium, Calcium, Magnesium
Q15: For Q. Nos., two statements are given – One labelled as Assertion (A) and the other labelled as Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below: (2024)
Assertion (A): Hydrogen gas is not evolved when zinc reacts with nitric acid.
Reason (R): Nitric acid oxidises the hydrogen gas produced to water and itself gets reduced.
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of the Assertion (A).
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A).
(c) Assertion (A) is true, but Reason (R) is false.
(d) Assertion (A) is false, but Reason (R) is true.
Ans: (a)
In this scenario, both the Assertion (A) and the Reason (R) are true. When zinc reacts with nitric acid, hydrogen gas is not produced because the nitric acid oxidizes any hydrogen gas formed into water while itself getting reduced. Thus, the reason correctly explains why hydrogen gas is not evolved, making option (a) the right choice.
Q16: What is a chemical reaction? Describe one activity each to show that a chemical change has occurred in which (i) a change of colour, and (ii) a change in temperature has taken place. (2024)
Ans: A chemical reaction involves the breaking and making of bonds between atoms to produce new substances. when reactant changes to products.
(i) Add lead nitrate solution to potassium iodide solution taken in a test tube. The colour changes from colourless solution to yellow ppt.
(ii) Calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) releasing a large amount of heat.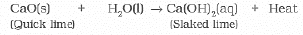
Q17: (i) Define a decomposition reaction. How can we say that (I) electrolysis of water, and (II) blackening of silver bromide when exposed to sunlight, are decomposition reactions? Mention the type of energy involved in each case.
(ii) The type of reactions in which (I) calcium oxide is formed, and (II) calcium hydroxide is formed are opposite reactions to each other. Justify this statement with the help of chemical equations. (2024)
Ans: (i) A reactant breaks down to give two or more products. A reaction which requires energy to split a compound or reactant in two or more simple substances.
(I) Water splits into hydrogen gas and oxygen gas.
Type of Energy: Electrical energy
(II) Silver bromide decomposes into silver and bromine
Type of Energy: Light energy
(ii) (I) Formation of calcium oxide: It is an endothermic reaction/decomposition reaction.
It is an endothermic reaction/decomposition reaction.
(II) Formation of calcium hydroxide: It is exothermic/combination reaction
It is exothermic/combination reaction
Q18: (a) Copper powder is taken in a china dish and heated over a burner. Name the product formed and state its colour. Write the chemical equation for the reaction involved. (2024)
OR
(b) Write a chemical equation for the chemical reaction that occurs when the aqueous solutions of barium chloride and sodium sulphate react together. Write the symbols of the ions present in the compound precipitated in the reaction.
Ans: (a) Copper Oxide
It’s colour is Black. OR(b)
OR(b)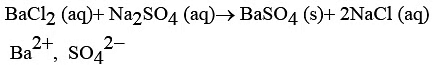
Q19: (A) Write the essential conditions for the following reaction to take place and name its types:
2AgCl → 2Ag + Cl2
(B) Complete the following chemical reaction in the form of a balanced equation:
(CBSE 2024)
Ans: (A) 
Sunlight is essential for the above reaction to take place. This is a decomposition reaction. Such reactions require energy either in the form of heat, light or electricity for breaking down the reactants. Silver chloride turns grey after its decomposition into silver and chlorine by sunlight. This reaction is used in black and white photography.
(B) 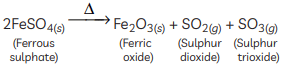
Previous Year Questions 2023
Q1: When aqueous solutions of potassium iodide and lead nitrate are mixed, an insoluble substance separates out. The chemical equation for the reaction involved is (2023)
(a) Kl + PbNO3 → Pbl + KNO3
(b) 2KI+ Pb(NO3)2 → Pbl2 + 2KNO3
(c) KI + Pb(NO3)2 → Pbl +KNO3
(d) Kl + PbNO3 → Pbl2 + KNO3
Ans: (b)
When potassium iodide (KI) and lead nitrate (Pb(NO3)2) are mixed, they react to form lead iodide (PbI2), which is an insoluble substance that precipitates out of the solution, and potassium nitrate (KNO3). The correct balanced equation for this reaction is 2KI+ Pb(NO3)2 → Pbl2 + 2KNO3. This shows that two potassium iodide molecules are needed to react with one lead nitrate molecule to produce one lead iodide and two potassium nitrate molecules, confirming option (b) as the correct answer.
Q2: The balanced chemical equation showing the reaction between quick lime and water is (2023)
(a) 2CaO + H2O → 2CaOH + H2 + Heat
(b) CaO + H2O → Ca(OH)2 + H2 + Heat
(c) CaO + H2O → Ca(OH)2 + Heat
(d) 2CaO + 3H2O → 2Ca(OH)3 + O2 + Heat
Ans: (c)
The balanced chemical equation for the reaction between quick lime (calcium oxide, CaO) and water CaO + H2O → Ca(OH)2 + Heat. In this reaction, quick lime reacts with water to form calcium hydroxide (Ca(OH)2), which is also known as slaked lime, and releases heat. This makes option (c) the correct answer, as it accurately represents the products and the heat released during the reaction.
Q3: Assertion (A): In the following reaction ZnO + C → Zn + CO
ZnO undergoes reduction.
Reason (R): Carbon is a reducing agent that reduces ZnO to Zn. (2023)
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A)
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is False.
(d) Assertion (A) is false, but Reason (R) is true.
Ans: (a)
Sol: The reaction in which oxygen is added or hydrogen is removed or loss of electrons takes place is called an oxidation reaction. In the reaction,
(i) Carbon is getting oxidised to carbon monoxide.
(ii) Zinc oxide is getting reduced to zinc.
Carbon is a reducing agent that reduces ZnO to Zn.
Q4: Assertion (A): The reaction of quick lime with water is an exothermic reaction.
Reason (R): Quicklime reacts vigorously with water releasing a large amount of heat. (2023)
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A)
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is False
(d) Assertion (A) is false, but Reason (R) is true
Ans: (a)
Sol: Reaction of quick lime (CaO) with water is an exothermic reaction because CaO reacts vigorously with water releasing a large amount of heat.
CaO(s) + H2O(I) → Ca(OH)2(ag) + Heat
Q5: (i) While electrolyzing water before passing the current some drops of an acid are added why? Name the gases liberated at the cathode and anode. Write the relationship between the volume of gas collected at the anode and the volume of gas collected at the cathode.
(ii) What is observed when silver chloride is exposed to sunlight? Give the type of reaction involved. (2023 C)
Ans:
(i) Acid is added to water before electrolysis to increase its conductivity. This allows the current to pass through the solution easily. Hydrogen gas is liberated at the cathode, while oxygen gas is liberated at the anode. The volume of gas collected at the anode is twice the volume of gas collected at the cathode. 2H2O(l) → 2H2(g) + O2(g)
(ii) When silver chloride is exposed to sunlight, it decomposes to form silver metal and chlorine gas. 2AgCl(s) → 2Ag(s) + Cl2(g) This is a photochemical decomposition reaction.
Q6: (a) Define a double displacement reaction.
(b) Write the chemical equation of a double displacement reaction which is also a (i) Neutralisation reaction and (ii) Precipitation reaction. Give justification for your answer. (2023)
Ans:
(a) The chemical reaction in which two reactants exchange ions to form two new compounds is called a double displacement reaction.
(b) (i) When an aqueous solution of an acid reacts with a base (alkali) by exchanging their ions/radicals to form salt and water as the only products, the reaction which takes place is called neutralisation reaction.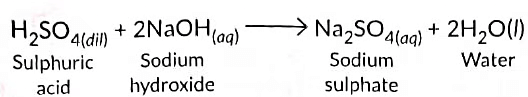
(ii) When the aqueous solutions of two ionic compounds react by exchanging their ions/radicals, to form two or more new compounds such that one of the products formed is an insoluble salt, and hence forms precipitate, the double displacement reaction is said to be precipitation reaction. When lead nitrate solution is mixed with potassium iodide solution, a yellow precipitate is formed. This reaction is a precipitation reaction and can be expressed as follows:
Q7: The emission of brown fumes in the given experimental set-up is due to:
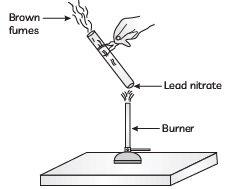
(a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
(b) thermal decomposition of lead nitrate which produces brown fumes of lead oxide.
(c) oxidation of lead nitrate forming lead oxide and nitrogen dioxide.
(d) oxidation of lead nitrate forming lead oxide and oxygen. (CBSE 2023)
Ans: (a)
When lead nitrate is heated, it undergoes thermal decomposition, resulting in the formation of lead oxide,PbO (a yellow solid), nitrogen dioxide NO2 (a brown gas), and oxygen, O2. The brown fumes observed in this setup are due to the release of nitrogen dioxide gas.
The reaction is as follows: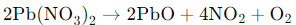
Thus, the correct answer is (a) thermal decomposition of lead nitrate which produces brown fumes of nitrogen dioxide.
Q8: In the experimental setup given below, it is observed that on passing the gas produced in the reaction in the solution ‘X’ the solution ‘X’ first turns milky and then colourless.
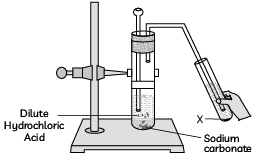
The option that justifies the given observation is that ‘X’ is aqueous calcium hydroxide and:
(a) it turns milky due to carbon dioxide gas liberated in the reaction and after some time it becomes colourless due to the formation of calcium carbonate.
(b) it turns milky due to the formation of calcium carbonate and on passing excess of carbon dioxide it becomes colourless due to the formation of calcium hydrogen carbonate which is soluble in water.
(c) it turns milky due to the passing of carbon dioxide through it. It turns colourless as on further passing carbon dioxide, sodium hydrogen carbonate is formed which is soluble in water.
(d) the carbon dioxide liberated during the reaction turns lime water milky due to the formation of calcium hydrogen carbonate and after some time, it turns colourless due to the formation of calcium carbonate which is soluble in water. (CBSE 2023)
Ans: (b)
When carbon dioxide is passed through aqueous calcium hydroxide (lime water), it initially reacts to form calcium carbonate, which is insoluble in water and causes the solution to turn milky: However, when excess carbon dioxide is passed through the solution, the calcium carbonate further reacts with the carbon dioxide and water to form calcium hydrogen carbonate, which is soluble in water, causing the solution to become clear again:
However, when excess carbon dioxide is passed through the solution, the calcium carbonate further reacts with the carbon dioxide and water to form calcium hydrogen carbonate, which is soluble in water, causing the solution to become clear again:
 Thus, the correct answer is (b).
Thus, the correct answer is (b).
Q9: Assertion (A): The colour of aqueous solution of copper sulphate turns colourless when a piece of lead is added to it.
Reason (R): Lead is more reactive than copper, and hence displaces copper from its salt solution.
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A)
(b) Both Assertion (A) and Reason (R) are true, but Reason (R) is not the correct explanation of the Assertion (A)
(c) Assertion (A) is true, but Reason (R) is False
(d) Assertion (A) is false, but Reason (R) is true (CBSE 2023)
Ans: (a)
Assertion (A): The color of the aqueous solution of copper sulfate turns colourless when a piece of lead is added to it. This is true. Copper sulfate solution is blue due to the presence of copper ions. When lead is added, it reacts with copper sulfate, displacing copper and forming lead sulfate, which is colourless in solution.
Reason (R): Lead is more reactive than copper and displaces copper from its salt solution. This is also true. Lead, being more reactive, displaces copper ions from copper sulfate, resulting in the precipitation of copper and the formation of colourless lead sulfate in the solution.
Since the reason correctly explains the assertion, the correct answer is (a)
Q10: (A) Identify the reducing agent in the following reactions:
(i) 4NH3 + 5O2 → 4NO + 6H2O
(ii) H2O + F2 → HF + HOF
(iii) Fe2O3 + 3CO → 2Fe + 3CO2
(iv) 2H2 + O2 → 2H2O
(B) Define a redox reaction in terms of gain or loss of oxygen.
Ans: (A) (i) NH3 is the reducing agent because it gets oxidised to NO by the removal of hydrogen and addition of oxygen. O2 has been reduced to H2O by the addition of hydrogen.
(ii) H2O is the reducing agent. Here, F2 gets reduced to HF (addition of hydrogen) and H2O gets oxidised to HOF (removal of hydrogen).
(iii) CO is the reducing agent. Here, CO has been oxidised to CO2 by the addition of oxygen. Fe2O3 has been reduced to Fe by the removal of oxygen.
(iv) H2 is the reducing agent as it gets oxidised to H2O by the addition of oxygen. O2 has been reduced to H2O by the addition of hydrogen.
(B) The reaction in which one element gets oxidised or addition of oxygen occurs and other element gets reduced or removal of oxygen occurs in other element is called redox reaction.
Example: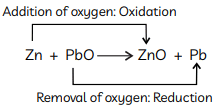
 | Get additional INR 200 off today with EDUREV200 coupon. | Avail Offer |
Previous Year Questions 2022
Q1: Sodium reacts with water to form sodium hydroxide and hydrogen gas. The balanced equation which represents the above reaction is (2022)
(a) Na(s) + 2H2O(l) → 2NaOH(aq) + 2H2(g)
(b) 2Na(s) + 2H2O(l) → 2NaOH(aq) +H2(g)
(c) 2Na(s) + 2H2O(l) → NaOH(aq) + 2H2(g)
(d) 2Na(s) + H2O(l) → 2NaOH(aq) + 2H2(g)
Ans: (b)
In the reaction between sodium (Na) and water (H2O), two sodium atoms react with two water molecules to produce two sodium hydroxide (NaOH) molecules and one hydrogen gas (H2) molecule. The balanced equation shows that two sodium atoms are needed for every two water molecules, which is why option (b) is correct. It accurately represents the conservation of mass in the reaction.
Q2: It is important to balance the chemical equations to satisfy the law of conservation of mass. Which of the following statements of the law is incorrect? (2022)
(a) The total mass of the elements present in the reactants is equal to the total mass of the elements present in the products.
(b) The number of atoms of each element remains the same, before and after a chemical reaction.
(c) The chemical composition of the reactants is the same before and after the reaction.
(d) Mass can neither be created nor can it be destroyed in a chemical reaction.
Ans: (c)
Sol: A balanced equation follows law of conservation of mass that means the total mass of reactants is equal to the total mass of products but the chemical composition of reactants does not remain same before and after the reaction.
Q3: C6H12O6(aq) + 6O2(g) → 6CO2(g) + 6H2O(I)
The above reaction is a/an (2022)
(a) Displacement reaction
(b) Endothermic reaction
(c) Exothermic reaction
(d) Neutralisation reaction
Ans: (c)
Sol: In the process of respiration, glucose combines with oxygen in cells of our body and provides energy. Thus, respiration is an exothermic process.
C6H12O6(aq) + 6O2(g) → 6CO2(g) + 6H2O(I) + Energy
Q4: Which of the following statements about the reaction given below are correct?
MnO2 + 4HCI → MnCl2 + 2H2O + Cl2
(i) HCL is oxidized to Cl2.
(ii) MnO2 is reduced to MnCl2.
(iii) MnCl2 acts as an oxidizing agent.
(iv) HCI acts as an oxidizing agent. (2022)
(a) (ii), (iii) and (iv)
(b) (i), (ii) and (iii)
(c) (i) and (ii) only
(d) (iii) and (iv) only
Ans: (c)
In the reaction, hydrochloric acid (HCl) loses electrons and is converted to chlorine gas (Cl2), so statement (i) is correct. Manganese dioxide (MnO2) gains electrons and is transformed into manganese chloride (MnCl2), making statement (ii) correct as well. However, MnCl2 does not act as an oxidizing agent; it is the reduced form of manganese. Therefore, the correct statements are (i) and (ii), which is why option (c) is the right choice.
Q5: Assertion (A): Burning of natural gas is an endothermic process.
Reason (R): Methane gas combines with oxygen to produce carbon dioxide and water. (2022)
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Ans: (d)
In this case, the Assertion (A) is false because the burning of natural gas (which is primarily methane, CH₄) is actually an exothermic process, meaning it releases heat. The Reason (R) is true because methane does combine with oxygen to produce carbon dioxide (CO2) and water (H2O) during combustion. Thus, option (d) is the correct choice, stating that the Assertion is false while the Reason is true.
Q6: Consider the following processes
I. Dilution of sulphuric acid
II. Sublimation of dry ice
III. Condensation of water vapours
IV. Dissolution of ammonium chloride in water
The endothermic process(es) is/are (2022)
(a) I and III
(b) Il only
(c) Ill only
(d) Il and IV
Ans: (d)
Sol: During sublimation of dry ice, heat is absorbed, so, it is an endothermic process. Dissolution of NH4CI in water is also an endothermic process.
Q7: When lead nitrate powder is heated in a boiling tube. we observe (2022)
(a) Brown fumes of nitrogen dioxide
(b) Brown fumes of lead oxide
(c) Yellow fumes of nitrogen dioxide
(d) Brown fumes of nitric oxide.
Ans: (a)
When lead nitrate (Pb(NO3)2) is heated, it decomposes and produces brown fumes of nitrogen dioxide (NO2). This happens because the lead nitrate breaks down into lead oxide (PbO), nitrogen dioxide (NO2), and oxygen (O2). The brown color of the fumes is characteristic of nitrogen dioxide, making option (a) the correct answer.
Q8: Assertion (A): Silver salts are used in black-and-white photography.
Reason (R): Silver salts do not decompose in the presence of light. (2022)
(a) Both (A) and (R) are true and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true but (R) is not the correct explanation of (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Ans: (c)
Sol: Silver salt (AgCl) are used in black and white photography. Silver salt (AgCl) is photosensitive compound, it decomposes into elemental chlorine (Cl2) and Ag(metal).
AgBr is also used as black and white photography.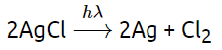
Q9: Mention with reason the colour changes observed when: (2022)
(A) Silver chloride is exposed to sunlight
(B) Copper powder is strongly heated in the presence of oxygen
(C) A piece of zinc is dropped in copper sulphate solution.
Ans:

AgCI decomposes on absorbing light energy.
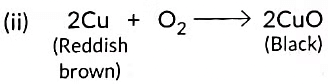
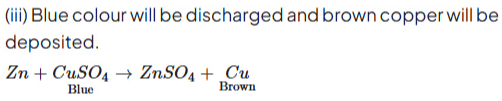
Copper metal undergoes oxidation. Zn displaces Cu from CuSO4 solution. Colour changes from blue to colourless.
Q10: A shining metal ‘M’, on burning gives a dazzling white flame and changes to a white powder ‘N’.
(a) Identify ‘M’ and ‘N’.
(b) Represent the above reaction in the form of a balanced chemical equation.
(c) Does ‘M’ undergo oxidation or reduction in this reaction? Justify. (2022)
Ans:
(a) ‘M’ is (Mg) Magnesium and ‘N’ is (MgO) Magnesium Oxide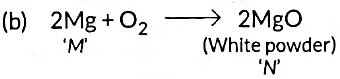
(c) ‘M’ undergoes oxidation in this reaction because Mg gain oxygen to form MgO.
Previous Year Questions 2021
Q1: What is a balanced chemical equation? (2021 C)
Ans: The equation which contains an equal number of atoms of each element on both sides of the arrow is called a balanced chemical equation.
Q2: Name the type of chemical reaction that takes place when quicklime is added to water. (2021 C)
Ans: The reaction between CaO and H2O to form Ca(OH)2 is an exothermic and combination reaction.
Q3: Give the chemical name of the reactants as well as the products of the following chemical equation: (2021 C)
HNO3 + Ca(OH)2 → Ca(NO3)2 + H2O
Ans: Reactants: Nitric acid, calcium hydroxide (slaked lime)
Products: Calcium nitrate, water
Q4: Assertion (A): Burning of natural gas is an endothermic process.
Reason (R): Methane gas combines with oxygen to produce carbon dioxide and water
(a) Both (A) and (R) are true, and (R) is the correct explanation of (A).
(b) Both (A) and (R) are true, and (R) is not the correct explanation of (A).
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true. (CBSE Term-1 2021)
Ans: (d)
Assertion (A): Burning of natural gas is an endothermic process. This is incorrect. Burning (or combustion) of natural gas, which mainly consists of methane, is an exothermic process, meaning it releases heat.
Reason (R): Methane gas combines with oxygen to produce carbon dioxide and water. This statement is correct. During combustion, methane reacts with oxygen to produce carbon dioxide and water as products.
Since the assertion is false, but the reason is true, the correct answer is (d) (A) is false but (R) is true.
| Also read: Important Equations and Definitions: Chemical Reactions and Equations |
Previous Year Questions 2020
Q1: In which of the following, the identity of the initial substance remains unchanged? (2020)
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Ans: (b)
Sol: Formation of crystals is a physical change while others are chemical change.
Q2: Assertion (A): The following is a balanced chemical equation for the action of steam on iron:
3Fe (s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g)
Reason (R): The law of conservation of mass holds good for a chemical equation. (2020)
(a) Both (A) and (R) are true and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Ans: (a)
In this question, both the Assertion (A) and the Reason (R) are true.
The balanced chemical equation 3Fe (s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g) correctly represents the reaction of steam with iron, forming iron(II,III) oxide (Fe3O4) and hydrogen gas (H2).
The Reason (R) states that the law of conservation of mass holds, meaning that mass is neither created nor destroyed in a chemical reaction, which is indeed reflected in the balanced equation. Therefore, option (a) is correct, as the Reason accurately explains the Assertion.
Q3: When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a (2020)
(a) Combination reaction
(b) Displacement reaction
(c) Decomposition reaction
(d) Double displacement reaction.
Ans: (d)
When hydrogen sulfide (H2S) is passed through a blue solution of copper sulfate (CuSO4), it forms a black precipitate of copper sulfide (CuS) while sulfuric acid (H2SO4) remains in the solution. This process involves the exchange of ions between the reactants, characteristic of a double displacement reaction. In this type of reaction, the ions from both compounds swap partners, which makes option (d) the correct answer.
Q4: In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution:
(A) Exchange of atoms takes place
(B) Exchange of ions takes place
(C) A precipitate is produced
(D) An insoluble salt is produced
The correct option is (2020)
(a) (B) and (D)
(b) (A) and (C)
(c) Only (B)
(d) (B), (C) and (D)
Ans: (d)
In a double displacement reaction, like the one between sodium sulfate and barium chloride, the ions in the reactants swap places. This results in the formation of an insoluble salt (barium sulfate), which is a solid that separates out (precipitate) from the solution. So, options (B), (C), and (D) are all correct because they describe the ion exchange and the formation of a precipitate.
Q5: Mention with reason the colour changes observed when:
(i) Silver chloride is exposed to sunlight.
(ii) copper powder is strongly heated in the presence of oxygen. (2020)
Ans:
AgCl decomposes on absorbing light energy.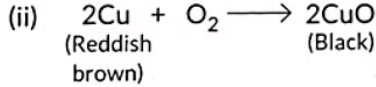
Copper metal undergoes oxidation.
Q6: If copper is kept open in the air, it slowly loses its shining brown surface and gains a green coating. It is due to the formation of (2020)
(a) CuSO4
(b) CuCO3
(c) Cu(NO3)2
(d) CuO
Ans: (b)
When copper is left exposed to air, it reacts with oxygen and carbon dioxide, leading to the formation of a green coating called copper carbonate (CuCO3). This green layer is a sign of corrosion and happens over time as the copper oxidizes, which is why option (b) is correct.
Q7: What happens when food materials containing fats and oils are left for a long time? List two observable changes and suggest three ways by which this phenomenon can be prevented. (CBSE 2020)
Ans: Food materials containing fats and oils change when left for a long time due to a process called rancidity. This occurs when air interacts with these substances, affecting their smell and taste. The observable changes include:
- The food develops an unpleasant smell.
- The taste of the food becomes off or stale.
To prevent rancidity, consider these methods:
- Vacuum packing to limit air exposure.
- Refrigeration to slow down oxidation.
- Storing food away from direct sunlight to reduce heat exposure.
Q8: In the electrolysis of water
(a) Name the gases liberated at the anode and cathode.
(b) Why is it that the volume of gas collected on one electrode is two times that on the other electrode?
(c) What would happen if dil. H2SO4 is not added to water? (2020)
Ans: (a) At anode: Oxygen gas is liberated. At cathode: Hydrogen gas is liberated.
(b) In the test tube covering the cathode, the amount of gas collected is double than that of the gas collected in the test tube covering the anode due to stochiometry.
2H2O → 2H2 + O2
(c)Without adding dilute sulphuric acid, water would not conduct electricity effectively, which would hinder the electrolysis process. As addition of a few drops of sulphuric acid make water a good conductor of electricity.
Q9: 1 g of copper powder was taken in a China dish and heated. What change takes place in healing? When hydrogen gas is passed over this heated substance, a visible change is seen in it. Give the chemical equations of reactions, the name and the colour of the products formed in each case. (2020)
Ans: When copper powder is heated in a China dish, the reddish brown surface of copper powder becomes coated with a black substance which is copper oxide.
When hydrogen gas is passed over CuO, the black coating on the surface turned reddish brown due to the formation of Cu.
Q10: A compound ‘A’ is used in the manufacture of cement. When dissolved in water, it evolves a large amount of heat and forms compound ‘B’.
(i) Identify A and B.
(ii) Write the chemical equation for the reaction of A with water.
(iii) List two types of reactions in which this reaction may be classified. (2020)
Ans: (i) Compound A is calcium oxide (CaO) and compound B is calcium hydroxide (Ca(OH)2).
(ii) The chemical equation for the reaction of A (calcium oxide) with water is:
CaO(s) + H2O(l) → Ca(OH)2(aq)
(iii) The reaction can be classified as a combination reaction and an exothermic reaction. It is a combination reaction because two substances combine to form a new compound, and it is exothermic because it evolves a large amount of heat.
Q11: Identify the type of each of the following reactions. Also, write a balanced chemical equation for each reaction.
(i) A reaction in which the reaction mixture becomes warm.
(ii) A reaction in which an insoluble substance is formed. (2020)
Ans: (i) The type of reaction in which the reaction mixture becomes warm is an exothermic reaction. An example of such a reaction is the combustion of methane (CH4):
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + heat
(ii) The type of reaction in which an insoluble substance is formed is a precipitation reaction. An example of such a reaction is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl):
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
In this reaction, silver chloride (AgCl) is formed as an insoluble substance, which precipitates out of the solution.
Q12: Lead nitrate solution is added to a test tube containing potassium iodide solution.
(a) Write the name and colour of the compound precipitated.
(b) Write the balanced chemical equation for the reaction involved.
(c) Name the type of this reaction justifying your answer. (2020)
Ans: (a) The compound precipitated is lead iodide (PbI2), which is yellow in color.
(b) The balanced chemical equation for the reaction is:
Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq)
(c) The type of this reaction is a precipitation reaction. In this reaction, a solid (precipitate) is formed when two solutions are mixed together. In this case, lead iodide is formed as a yellow precipitate.
Q13: Study the figure given below and answer the following questions:
(A) Name the process depicted in the diagram.
(B) Write the composition of gases collected at the anode and cathode.
(C) Write the balanced chemical equation of the reaction taking place in this case.
(D) The reaction does not take place if a few drops of dilute sulphuric acid are not added to water. Why? (CBSE 2020)
Ans: (A) Electrolytic decomposition of water/ electrolysis of water.
(B) The gas collected at cathode is hydrogen which is double the volume of oxygen collected at anode.
(C) The balanced chemical equation for the reaction is: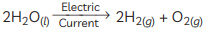
(D) The reaction does not occur without dilute sulphuric acid because:
- Water is a poor conductor of electricity.
- Adding sulphuric acid improves conductivity, allowing the reaction to proceed.
Previous Year Questions 2019
Q1: Translate the following statement into a balanced chemical equation :
“Barium chloride reacts with aluminium sulphate to give aluminium chloride and barium sulphate.” (CBSE 2019)
Ans: 3BaCl2 + Al2(SO4)3 → 2AICI3 + 3BaSO4
Q2: What is observed after about 1 hour of adding the strips of copper and aluminium separately to the ferrous sulphate solution filled in two beakers? Name the reaction if any change in colour is noticed. Also, write a chemical equation for the reaction. (CBSE 2019)
Ans:
Cu(s) + FeSO₄(aq) → No change will take place
Copper is less reactive than Fe, so Cu cannot displace iron from a ferrous sulphate solution. Hence, No reaction will take place.
2 Al(s) + 3 FeSO₄(aq) → Al₂(SO₄)₃ + 3 Fe(s) (Displacement reaction)
When Al is added to a FeSO₄(aq) solution, the green colour of FeSO₄(aq) disappears and the Fe is seen setting down as the reaction occurs. Al being higher in the reactivity series displaces the Fe in FeSO₄.
Q3: A student wants to study a decomposition reaction by taking ferrous sulphate crystals. Write two precautions he must observe while performing the experiment. (CBSE 2019)
Ans: (i) Test tube should be dried properly.
(ii) Hold the test tube in a test tube holder.
Q4: 2 g of silver chloride is taken in a China dish, and the China dish is placed in sunlight for some time. What will be your observation in this case? Write the chemical reaction involved in the form of a balanced chemical equation. Identify the type of chemical reaction. (Delhi 2019)
Ans: When silver chloride (AgCl) is exposed to sunlight, it undergoes a decomposition reaction and gets converted into silver (Ag) and chlorine gas (Cl2). The balanced chemical equation for this reaction is:
2AgCl(s) → 2Ag(s) + Cl2(g)
The observation in this case would be that the white silver chloride gradually turns grayish-white or silver-colored due to the formation of silver, and the chlorine gas may be seen as a pale yellowish-green gas. This is an example of a photodecomposition reaction, where light energy is used to break down the compound into its elements.
Q5: Identify the type of reactions taking place in each of the following cases and write the balanced chemical equation for the reactions.
(a) Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(b) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide. (CBSE 2019)
Ans: (a) The type of reaction taking place is a single displacement reaction. Zinc (Zn) displaces silver (Ag) from silver nitrate (AgNO3) to form zinc nitrate (Zn(NO3)2) and silver (Ag). The balanced chemical equation for this reaction is:
Zn(s) + 2AgNO3(aq) → Zn(NO3)2(aq) + 2Ag(s)
(b) The type of reaction taking place is a double displacement reaction or a precipitation reaction. Potassium iodide (KI) reacts with lead nitrate (Pb(NO3)2) to produce potassium nitrate (KNO3) and lead iodide (PbI2). The balanced chemical equation for this reaction is:
2KI(aq) + Pb(NO3)2(aq) → 2KNO3(aq) + PbI2(s)
Q6: When potassium iodide solution is added to a solution of lead (II) nitrate in a test tube, a precipitate is formed.
(a) What is the colour of this precipitate? Name the compound precipitated.
(b) Write the balanced chemical equation for this reaction.
(c) List two types of reactions in which this reaction can be placed. (2019)
Ans: (a) The color of the precipitate formed is yellow. The compound precipitated is lead iodide (PbI2)
(b) The balanced chemical equation for this reaction is:
2KI(aq) + Pb(NO3)2(aq) → PbI2(s) + 2KNO3(aq)
(c) The two types of reactions in which this reaction can be placed are a double displacement reaction and a precipitation reaction. In a double displacement reaction, the positive and negative ions of two compounds switch places to form new compounds. In a precipitation reaction, a solid precipitate is formed when two solutions are mixed together.
Q7: 2 g of ferrous sulfate crystals are heated in a dry boiling tube. (a) List any two observations. (b) Name the type of chemical reaction taking place. (c) Write a balanced chemical equation for the reaction and name the products formed. (Al 2019, Board Term 1, 2017, 2016)
Ans: (a) Two observations during the heating of ferrous sulfate crystals are:
1. The crystals lose water and become anhydrous.
2. The color of the crystals changes from green to a reddish-brown color.
(b) The type of chemical reaction taking place is a thermal decomposition reaction.
(c) The balanced chemical equation for the reaction is:
FeSO4⋅7H2O(s) → Fe2O3(s) + SO2(g) + H2O(g)
The products formed are ferric oxide (Fe2O3), sulfur dioxide (SO2), and water (H2O).
Q8: You might have noted that when copper powder is heated in a China dish, the reddish-brown surface of copper powder becomes coated with a black substance.
(a) Why has this black substance formed?
(b) What is the black substance?
(c) Write the chemical equation of the reaction that takes place.
(d) How can the black coating on the surface be turned reddish-brown? (Al 2019)
Ans: (a) The black substance is formed because copper reacts with oxygen in the air to form copper oxide.
(b) The black substance is copper oxide (CuO).
(c) The chemical equation for the reaction is:
2Cu(s) + O2(g) → 2CuO(s)
(d) The black coating on the surface can be turned reddish-brown by reducing it back to copper. This can be done by passing hydrogen gas over the hot copper oxide. The chemical equation for this reaction is:
CuO(s) + H2(g) → Cu(s) + H2O(g)
The reduction reaction converts the black copper oxide back to reddish-brown copper.
Q9: (A) Design an activity to demonstrate the decomposition reaction of lead nitrate.
(B) Draw a labelled diagram of the experimental set-up. List two main observations.
(C) Write a balanced chemical equation for the reaction stating the physical state of the reactant and the products. (CBSE 2019)
Ans: (A) Take a small amount of lead nitrate powder in a boiling tube. Hold the boiling tube with a pair of tongs and heat it over the flame first gently and then strongly.
(B) Labelled diagram of the experimental setup: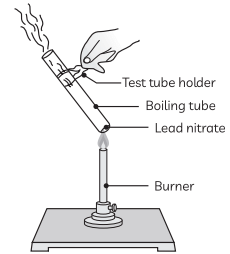
Two main observations:
(i) We observe emission of brown fumes of a gas which is nitrogen dioxide.
(ii) The white colour of lead nitrate changes to yellow colour as lead oxide is formed.
(C) Balanced equation: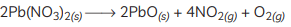
Previous Year Questions 2018
Q1: Decomposition reactions require energy either in the form of heat or light or electricity to break down the reactants. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light, and electricity. (2018)
Ans: Decomposition reaction with heat energy supplied:
ZnCO3(s) → ZnO(s) + CO2(g)
Decomposition reaction with light energy supplied:
2H2O2(l) → 2H2O(l) + O2(g)
Decomposition reaction with electrical energy supplied:
2AgCl(s) → 2Ag(s) + Cl2(g)
Previous Year Questions 2017
Q1: Take 3 g of barium hydroxide in a test tube. Now add about 2 g of ammonium chloride and mix the contents with the help of a glass rod. Now touch the test tube from outside.
(i) What do you feel about touching the test tube?
(ii) State the inference about the type of reaction that occurred.
(iii) Write the balanced chemical equation of the reaction involved. (Board Term 1, 2017)
Ans: (i) On touching the test tube from outside, you will feel the test tube becoming cold.
(ii) The inference about the type of reaction that occurred is that it is an endothermic reaction. In endothermic reactions, heat is absorbed from the surroundings, resulting in a decrease in temperature
(iii) The balanced chemical equation for the reaction is:
Ba(OH)2(s) + 2NH4Cl(s) → BaCl2(aq) + 2NH3(g) + 2H2O(l)
Q2: (a) Can a displacement reaction be a redox reaction? Explain with the help of an example.
(b) Write the type of chemical reaction in the following:
(i) Reaction between an acid and a base
(ii) Rusting of iron. (Board Term I, 2017)
Ans: (a) Consider the following displacement reaction:
Zn(s)+ CuSO4(aq) → ZnSO4(aq) + Cu(s)
Here, Zn has changed into ZnSO4 (i.e., Zn2+ ions) by loss of electrons. Hence, Zn has been oxidised. CuSO4 (i.e., Cu2+) has changed into Cu by gain of electrons. Hence, CuSO4 has been reduced. Thus, the above reaction is a displacement reaction as well as a redox reaction.
(b) (i) Neutralisation reaction
(ii) Oxidation reaction.
Previous Year Questions 2016
Q1: Name the type of chemical reaction represented by the following equation:
(i) CaO + H2O → Ca(OH)2
(ii) 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4
(iii) 2FeSO4 + Heat → Fe2O3 + SO2 + SO3 (Board Term 1, 2016)
Ans: (i) The type of chemical reaction represented by the equation CaO + H2O → Ca(OH)2 is a combination or synthesis reaction.
(ii) The type of chemical reaction represented by the equation 3BaCl2 + Al2(SO4)3 → 2AlCl3 + 3BaSO4 is a double displacement or precipitation reaction.
(iii) The type of chemical reaction represented by the equation 2FeSO4 + Heat → Fe2O3 + SO2 + SO3 is a thermal decomposition reaction.
Q2: (a) A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction. (NCERT Exemplar)
(b) Ferrous sulfate, when heated, decomposes with the evolution of a gas having a characteristic odor of burning sulfur. Write the chemical reaction involved and identify the type of reaction. (Board Term 1, 2016)
Ans: (a) The chemical reaction involved is a double displacement reaction or a precipitation reaction. The reaction between potassium chloride (KCl) and silver nitrate (AgNO3) produces silver chloride (AgCl), which is an insoluble white substance. The balanced chemical equation for this reaction is:
KCl(aq) + AgNO3(aq) → AgCl(s) + KNO3(aq)
(b) The chemical reaction involved is a thermal decomposition reaction. Ferrous sulfate (FeSO4) when heated decomposes to form ferric oxide (Fe2O3), sulfur dioxide (SO2), and water (H2O). The balanced chemical equation for this reaction is:
FeSO4(s) → Fe2O3(s) + SO2(g) + H2O(g)
| Also read: Important Equations and Definitions: Chemical Reactions and Equations |
Previous Year Questions 2015
Q1: What can be seen when a strip of copper metal is placed in a solution of silver nitrate? (CBSE 2015)
Ans: When a strip of copper metal is placed in a solution of silver nitrate, copper displaces silver from silver nitrate solution as copper is a more reactive metal than silver. Copper nitrate is formed with a shiny greyish white deposit of silver on the copper strip.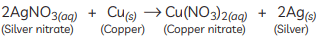
Q2: What is a reduction reaction? Identify the substances that are oxidised and the substances that are reduced in the following reactions. (A) Fe2O3 + 2Al → Al2O3 + 2Fe
(B) 2PbO + C → 2Pb + CO2 (CBSE 2015)
Ans: A reduction reaction is a reaction in which hydrogen is added to a substance or oxygen is removed from a substance.
(A) In this reaction, Fe2O3 is losing oxygen and forming Fe, whereas Al is gaining oxygen and forming Al2O3. Therefore, Fe2O3 is getting reduced and Al is getting oxidised.
(B) In this reaction, PbO is losing oxygen and forming Pb whereas C is gaining oxygen and forming CO. Therefore, PbO is getting reduced and C is getting oxidised.
Previous Year Questions 2012
Q1: Name and state the law that is kept in mind when we balance chemical equations. (CBSE 2012)
Ans: The law which should be kept in mind when we balance chemical equations is the law of conservation of mass which states that “Matter can neither be created nor be destroyed”. It means that the total mass of atoms of reactants is equal to total mass of atoms of products, as atoms can neither be created nor be destroyed.